Grantee Research Project Results
2010 Progress Report: Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
EPA Grant Number: R832415C004Subproject: this is subproject number 004 , established and managed by the Center Director under grant R832415
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Rochester PM Center
Center Director: Oberdörster, Günter
Title: Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
Investigators: Oberdörster, Günter , Elder, Alison C.P. , Oakes, David , Couderc, Jean-Philippe , Phipps, Richard , Gelein, Robert , Eberly, Shirley
Current Investigators: Oberdörster, Günter , Elder, Alison C.P. , Couderc, Jean-Philippe , Phipps, Richard , Gelein, Robert , Kreyling, Wolfgang , Oakes, David
Institution: University of Rochester , GSF-National Research Center for Environment and Health
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
Project Period Covered by this Report: June 30, 2009 through July 1,2010
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
Progress Summary:





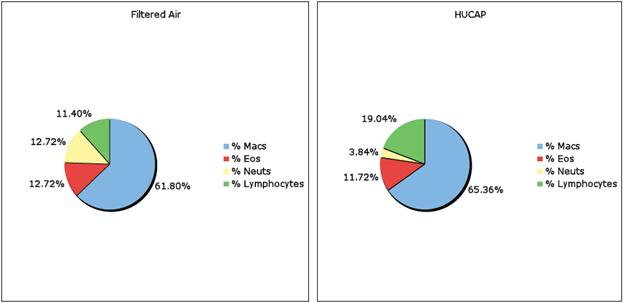
| Total Cell | % AM | % PMN | % Lymph | % Viable | Protein | LDH | B-Gluc | ||||
| 4 Day 2-w xSham | 6 wk | 1 wk-Sham | Ave | 0.114 | 98.43 | 0.00 | 0.51 | 74.74 | 0.25 | 70.98 | 0.07 |
| St. Dev | 0.03 | 1.84 | 0.00 | 0.18 | 4.86 | 0.048 | 11.12 | 0.006 | |||
| 4 Day 2x4D/4Hr | 6 wk | 1 x4D/4Hr | Ave | 0.253 | 97.62 | 0.819 | 2.02 | 76.95 | 0.239 | 59.22 | 0.135 |
| St. Dev | 0.288 | 0.84 | 0.43 | 0.66 | 10.61 | 0.081 | 10.62 | 0.132 | |||
| 4 Day 2wk-Sham | 6 wk | 1 x4D/4Hr | Ave | 0.076 | 98.08 | 0.291 | 0.85 | 76.44 | 0.384 | 69.59 | 0.068 |
| St. Dev | 0.021 | 0.88 | 0.11 | 0.14 | 16.34 | 0.139 | 6.66 | 0.014 | |||
| 56 Day 2x4D/4Hr | 6 wk | 1 x4D/4Hr | Ave | 0.231 | 99.11 | 0.18 | 0.71 | 86.56 | 0.302 | 68.1 | 0.167 |
| St. Dev | 0.08 | 0.24 | 0.03 | 0.22 | 3.98 | 0.049 | 10.52 | 0.01 |
| BRAIN | BRAIN | BRAIN | ||||||
| LUNG | LIVER | KIDNEY | SPLEEN | OLF. BULB | CERE | CERE. CORTEX | ||
| Sample Conc. (ppb) | Sample Conc. (ppb) | Sample Conc. (ppb) | Sample Conc. (ppb) | Sample Conc. (ppb) | Sample Conc. (ppb) | Sample Conc. (ppb) | ||
| 56 Day 2 x4D/4Hr | Ave | 27330 | 25.14 | 40.48 | 21.73 | 447.23 | 54.29 | 13.88 |
| SD | 1896.6 | 32.11 | 36.2 | 6.47 | 278.3 | 26.78 | 5.85 | |
| 4 Day 2 x4D/4Hr | Ave | 21053 | 167.05 | 220.05 | 30.25 | 289.03 | 35.48 | 7.93 |
| SD | 1342.1 | 70.22 | 80.97 | 9.32 | 104.19 | 27.24 | 2.17 |
Journal Articles on this Report : 9 Displayed | Download in RIS Format
| Other subproject views: | All 62 publications | 49 publications in selected types | All 43 journal articles |
|---|---|---|---|
| Other center views: | All 191 publications | 157 publications in selected types | All 144 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Bruske I, Hampel R, Socher MM, Ruckerl R, Schneider A, Heinrich J, Oberdorster G, Wichmann H-E, Peters A. Impact of ambient air pollution on the differential white blood cell count in patients with chronic pulmonary disease. Inhalation Toxicology 2010;22(3):245-252. |
R832415 (2010) R832415 (2011) R832415 (Final) R832415C002 (2010) R832415C002 (2011) R832415C004 (2010) R832415C004 (2011) R827354 (Final) |
Exit |
|
|
Hildebrandt K, Ruckerl R, Koenig W, Schneider A, Pitz M, Heinrich J, Marder V, Frampton M, Oberdorster G, Wichmann HE, Peters A. Short-term effects of air pollution: a panel study of blood markers in patients with chronic pulmonary disease. Particle and Fibre Toxicology 2009;6:25. |
R832415 (2009) R832415 (2010) R832415 (2011) R832415 (Final) R832415C002 (2009) R832415C002 (2010) R832415C002 (2011) R832415C003 (2010) R832415C003 (2011) R832415C004 (2010) R832415C004 (2011) |
Exit Exit Exit |
|
|
Oberdorster G, Stone V, Donaldson K. Toxicology of nanoparticles: a historical perspective. Nanotoxicology 2007;1(1):2-25. |
R832415 (2007) R832415 (2008) R832415 (2010) R832415 (2011) R832415 (Final) R832415C004 (2006) R832415C004 (2007) R832415C004 (2010) R832415C004 (2011) |
Exit Exit |
|
|
Oberdorster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? Journal of Nanoscience and Nanotechnology 2009;9(8):4996-5007. |
R832415 (2009) R832415 (2010) R832415 (2011) R832415 (Final) R832415C004 (2009) R832415C004 (2010) R832415C004 (2011) |
Exit |
|
|
Oberdorster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. Journal of Internal Medicine 2010;267(1):89-105. |
R832415 (2009) R832415 (2010) R832415 (2011) R832415 (Final) R832415C004 (2009) R832415C004 (2010) R832415C004 (2011) |
Exit Exit Exit |
|
|
Pui DYH, Qi C, Stanley N, Oberdorster G, Maynard A. Recirculating air filtration significantly reduces exposure to airborne nanoparticles. Environmental Health Perspectives 2008;116(7):863-866. |
R832415 (2007) R832415 (2008) R832415 (2010) R832415 (2011) R832415 (Final) R832415C004 (2006) R832415C004 (2010) R832415C004 (2011) |
|
|
|
Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, Oberdorster G. Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. Journal of Toxicology and Environmental Health, Part A 2010;73(5-6):445-461. |
R832415 (2010) R832415 (2011) R832415 (Final) R832415C004 (2010) R832415C004 (2011) R832415C005 (2010) R832415C005 (2011) |
Exit |
|
|
Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, Oberdorster G, Kreyling WG. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environmental Health Perspectives 2007;115(5):728-733. |
R832415 (2007) R832415 (2008) R832415 (2010) R832415 (2011) R832415 (Final) R832415C004 (2006) R832415C004 (2007) R832415C004 (2010) R832415C004 (2011) |
|
|
|
Zareba W, Couderc JP, Oberdorster G, Chalupa D, Cox C, Huang L-S, Peters A, Utell MJ, Frampton MW. ECG parameters and exposure to carbon ultrafine particles in young healthy subjects. Inhalation Toxicology 2009;21(3):223-233. |
R832415 (2008) R832415 (2009) R832415 (2010) R832415 (2011) R832415 (Final) R832415C002 (2010) R832415C002 (2011) R832415C003 (2010) R832415C003 (2011) R832415C004 (2009) R832415C004 (2010) R832415C004 (2011) R827354 (Final) |
Exit |
Supplemental Keywords:
Health, RFA, Scientific Discipline, Air, PHYSICAL ASPECTS, Health Risk Assessment, Physical Processes, Risk Assessments, particulate matter, Toxicology, animal model, atmospheric particles, ambient particle health effects, exposure, atmospheric aerosol particles, PM, ultrafine particulate matter, atmospheric particulate matter, inhalation toxicology, acute cardiovascular effects, cardiovascular disease, human health riskRelevant Websites:
None.Progress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832415 Rochester PM Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832415C001 Characterization and Source Apportionment
R832415C002 Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
R832415C003 Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
R832415C004 Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
R832415C005 Ultrafine Particle Cell Interactions In Vitro: Molecular Mechanisms Leading To Altered Gene Expression in Relation to Particle Composition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2011 Progress Report
- 2009 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
43 journal articles for this subproject
Main Center: R832415
191 publications for this center
144 journal articles for this center
