Grantee Research Project Results
2009 Progress Report: Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
EPA Grant Number: R832415C004Subproject: this is subproject number 004 , established and managed by the Center Director under grant R832415
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Rochester PM Center
Center Director: Oberdörster, Günter
Title: Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
Investigators: Oberdörster, Günter , Elder, Alison C.P. , Oakes, David , Couderc, Jean-Philippe , Phipps, Richard , Gelein, Robert , Kreyling, Wolfgang
Current Investigators: Oberdörster, Günter , Elder, Alison C.P. , Couderc, Jean-Philippe , Phipps, Richard , Gelein, Robert , Kreyling, Wolfgang , Oakes, David
Institution: University of Rochester , GSF-National Research Center for Environment and Health
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
Project Period Covered by this Report: October 1, 2008 through September 30,2009
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
The objective of the Core 4 studies is to correlate physico-chemical particle characteristics (from Core 1 measurements) with pulmonary and cardiovascular endpoints following exposure of animals to inhaled ambient concentrated ultrafine/fine particles, inhaled freshly generated exhaust particles from low and ultralow diesel fuel, and intratracheally administered ultrafine and fine ambient particles from different sites and sources. Effects measurements will take into account endpoints determined in the epidemiological (Core 2) and clinical (Core 3) studies and coordinate mechanistic evaluations with Core 5 in vitro studies. In addition, effects on the CNS will also be assessed.
Progress Summary:
Exposures of R6/2 Mice to Concentrated Ambient Ultrafine Particle-Containing Aerosols
Based on previous studies from our group demonstrating that inhaled poorly soluble laboratory-generated UFP travel to the brain and cause oxidative stress and inflammation in those regions where particles accumulate (Elder et al., 2006; Oberdörster et al., 2002, 2004), we hypothesized that ambient UFP can induce similar effects, particularly in an animal model that exhibits early-onset neurodegeneration. Transgenic R6/2 mice express a mutant human huntingtin protein (Htt) containing 105-150 polyglutamine (polyQ) repeats and are the best characterized and most widely used of the Huntington’s disease (HD) animal models. Aggregated Htt is abundant throughout the brain in these mice and they exhibit subtle motor deficits as early as one month of age; death occurs within three to four months (Mangiarini et al., 1996).
HD and other neurodegenerative diseases have in common abnormal protein folding and aggregation (e.g. Alzheimer’s, Creutzfeldt-Jakob, Parkinson’s). However, the significance of protein aggregation in these diseases is not entirely clear, as there is debate regarding whether it is the aggregated proteins or the misfolded monomers that are toxic. Nonetheless, recent acellular assays have shown that particles of different sizes, shapes, and surface chemistries can induce the unfolding and subsequent fibrillation (aggregation) of proteins (Linse et al., 2007). We hypothesized that the UFP present in ambient aerosols could be translocated to the brain, potentiate the aggregation of Htt, and accelerate the locomotor deficits in exposed R6/2 mice.
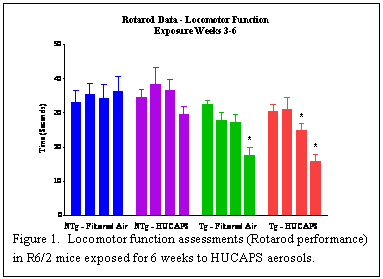
We exposed the R6/2 mice, starting at 5-7 days of age, to concentrated ambient UFP-containing (HUCAPS) aerosols for 4 hrs/day, 5 days/week, for a total of 6 weeks in whole-body exposure chambers. Starting 1 week after weaning, we conducted weekly evaluations of locomotor function (Rotarod) for a total of 4 weeks. Statistical analyses revealed that, unlike nontransgenic mice, the Rotarod performance of the transgenic mice declined over time. For those mice exposed to HUCAPS aerosols, the performance was significantly lower by the third week of testing, whereas for filtered air-exposed mice, performance did not drop significantly until the fourth testing week (Figure 1). The mice were euthanized 24-48 hrs after the last exposure, with one group being used for evaluations of lung inflammatory responses and one used to collect tissue samples for brain histopathological analyses. Serial coronal sections of brain tissue will be evaluated for striatal atrophy and huntingtin protein (Htt) expression and aggregation. Other tissues have also been saved so that we can examine Htt aggregation as a result of exposure in other tissues (e.g., lung, heart, pancreas).
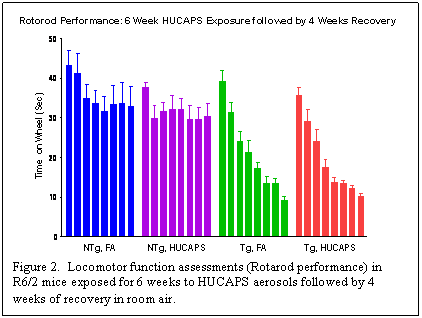
Another cohort of pups was exposed to HUCAPS aerosols or filtered air for 6 weeks and then recovered for 4 weeks. As with the study described above, locomotor function testing using the Rotarod system was done. In addition, their performance during the recovery period in a beam-break apparatus was evaluated to assess additional aspects of locomotor function, such as travelled distance and jumping. The mice were euthanized at the end of the 4-week recovery period and the same endpoints evaluated in brain tissue, lavage fluid, and blood. Statistical analyses, both of the Rotarod performance times (shown below in Figure 2) and of data from the beam-break system are currently ongoing.
Future Activities:
We will continue our studies to evaluate the effects of inhaled ambient ultrafine particles on the CNS. Specifically, we plan to follow up on our findings of the induction of persistent neuro-inflammation following exposure to UFP at an early age.
PM samples (size selective) collected from different source-specific sites by our Core 1 investigators will also be tested in our animal studies to determine their potential to induce pulmonary and systemic inflammatory responses following intratracheal instillation in rats. These studies are done in sequence with Core 1 (detailed physicochemical characterization) and Core 5 (in vitro ranking of oxidative stress inducing potential) studies to assess their in vivo-in vitro correlations and to establish hypotheses about source-specific mechanisms to be tested in future studies.
Journal Articles on this Report : 3 Displayed | Download in RIS Format
| Other subproject views: | All 62 publications | 49 publications in selected types | All 43 journal articles |
|---|---|---|---|
| Other center views: | All 191 publications | 157 publications in selected types | All 144 journal articles |
Supplemental Keywords:
Health, RFA, Scientific Discipline, Air, PHYSICAL ASPECTS, Health Risk Assessment, Physical Processes, Risk Assessments, particulate matter, Toxicology, animal model, atmospheric particles, ambient particle health effects, exposure, atmospheric aerosol particles, PM, ultrafine particulate matter, atmospheric particulate matter, inhalation toxicology, acute cardiovascular effects, cardiovascular disease, human health riskProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832415 Rochester PM Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832415C001 Characterization and Source Apportionment
R832415C002 Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
R832415C003 Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
R832415C004 Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
R832415C005 Ultrafine Particle Cell Interactions In Vitro: Molecular Mechanisms Leading To Altered Gene Expression in Relation to Particle Composition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2011 Progress Report
- 2010 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
43 journal articles for this subproject
Main Center: R832415
191 publications for this center
144 journal articles for this center