Grantee Research Project Results
Final Report: Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
EPA Grant Number: R832415C004Subproject: this is subproject number 004 , established and managed by the Center Director under grant R832415
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Rochester PM Center
Center Director: Oberdörster, Günter
Title: Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
Investigators: Oberdörster, Günter , Elder, Alison C.P. , Couderc, Jean-Philippe , Phipps, Richard , Gelein, Robert , Kreyling, Wolfgang , Oakes, David
Institution: University of Rochester , GSF-National Research Center for Environment and Health
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
The animal studies were designed to be complementary to the epidemiological and clinical studies and further test hypotheses derived from those studies, but also to explore novel areas of PM research such as effects on the brain, on the developing organism, or the impact of using cleaner fuel on diesel exhaust-induced health effects. Thus, response measurements took into account endpoints determined in the epidemiological (Core 2) and clinical (Core 3) studies. In addition, based on our earlier findings of translocation of inhaled UFP from nasal deposits to the brain, effects on the CNS were also assessed.
Summary/Accomplishments (Outputs/Outcomes):
Figure 1.
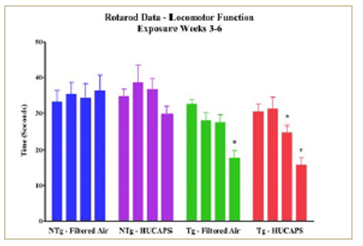
fIGURE 2.
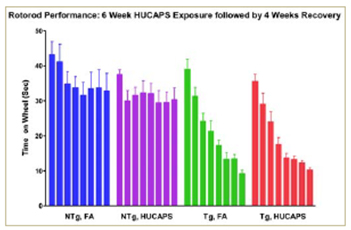
Figure 3.
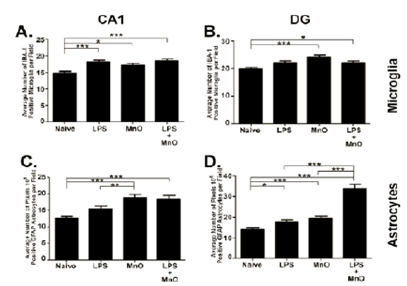
Figure 4.
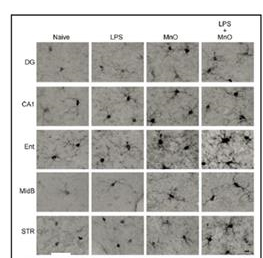
Figure 5. IBA-1-positive microglia in 3xTg-AD
exposure to Mn oxixde UFP +/- LPS. Bar, 10 µm.
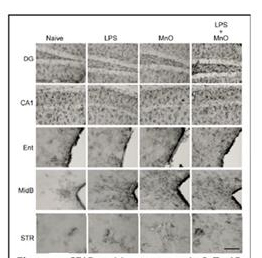
Figure 6. FGAP-positive astrocytes in 3xTg-AD
mouse brain regions two months following
exposure to Mn oxide UFP +/- LPS. Bar. 10 µm.
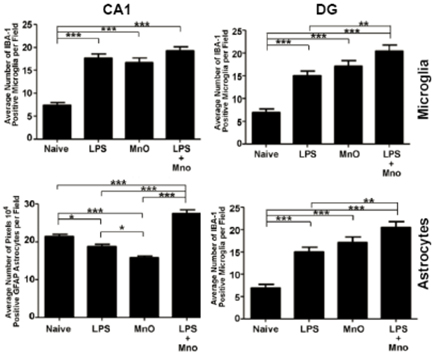
Figure 7.
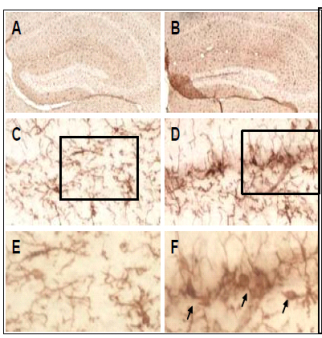 | Hippocampal sections were stained with Iba-1 to detect microgial cells. The images depict a robust microgial activation in the dentate gyrus of the hippocampus following neonatal UFP exposure. The upper panels (A, B) represent images of the dentate gyrus region taken at 2x magnification. Following UFP exposure during development, we observed increased numbers of microglial cells and enhanced Iba-1 staining in all layers of the dentate gyrus (B,D,F). The images depicted in panels C and D were captured from the hilar region of the dentate gyrus at 20x magnification. Panels E and F represent 40x images of the regions in the granule cell layer as outlined by the boxes in C and D. THe cells residing in corntrol tissue (C and E) displayed the classical ramified morphology of resting microglia. In contrast, the several cells shown in the dentate gyrus from exposed animals exhibited a more ameboid morphology (F, arrows) and increased intensity of Iba-1 (D,F), which are features consistent with microglial activation. |
Conclusions:
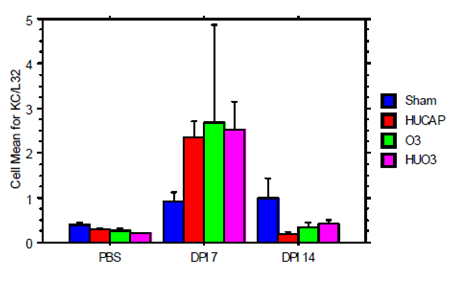
Figure 9. mRNA Abundance of Kc Day 182 - infected with influenza A.
- Six-hour exposure of obese diabetic and lean control rats to diesel exhaust (low and ultralow sulfur fuel) in a mobile lab on highway induced significant decreases in serum leptin; non-significant trend of higher levels in serum and lung lavage, of inflammatory cytokines; trend of lower GFAP level in brain regions and higher levels of inflammatory cytokines in olfactory bulb (non-significant).
- Four-week exposure of obese diabetic and lean rats to concentrated ambient UFP showed increased lung lavage protein and LDH levels at 3 days of exposure but not at the end of 4-week exposure, indicating potential adaptive mechanisms. Platelet number decreased in both rat types at the end of 4-week exposure. The obese diabetic rats are not more sensitive than their lean litter mates.
- Four-week exposure to concentrated ambient UFP resulted in a disruption of iron homeostasis in obese diabetic rats indicting higher baseline levels of oxidative stress.
- Obese diabetic rats have higher leptin levels in serum and lavage fluid.
- Concentrated UF particle exposure in an early onset neurodegeneration mouse model may accelerate decline in locomotor function.
- Twelve-day ultrafine Mn-oxide exposure in a mouse model of Alzheimer's Disease induced enhanced activation of the innate immune system in hippocampus and dentate gyrus which persisted through two months post-exposure.
- Neonatal mice inhalation exposure to gold nanoparticles confirmed their translocation to extrapulmonary tissues, including the olfactory bulb of the CNS.
- Eight days of neonatal mouse inhalation exposure to concentrated ambient UF particles induced microglial activation in the dentate gyrus of the hippocampus, which was still present in adulthood.
- Neonatal mouse inhalation exposure to concentrated ambient UF particles in combination with ozone sensitizes the lung to later life viral challenges.
- Early life exposure of mice with a pesticide-based Parkinson's disease phenotype to concentrated UFP enhances locomotor reduction with concurrent damage to striatum and midbrain.
References:
Journal Articles:
No journal articles submitted with this report: View all 62 publications for this subprojectSupplemental Keywords:
Health, RFA, Scientific Discipline, Air, PHYSICAL ASPECTS, Health Risk Assessment, Physical Processes, Risk Assessments, particulate matter, Toxicology, animal model, atmospheric particles, exposure, PM, ambient particle health effects, atmospheric aerosol particles, inhalation toxicology, ultrafine particulate matter, atmospheric particulate matter, cardiovascular disease, human health risk, acute cardiovascular effectsProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832415 Rochester PM Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832415C001 Characterization and Source Apportionment
R832415C002 Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
R832415C003 Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
R832415C004 Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
R832415C005 Ultrafine Particle Cell Interactions In Vitro: Molecular Mechanisms Leading To Altered Gene Expression in Relation to Particle Composition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2011 Progress Report
- 2010 Progress Report
- 2009 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
43 journal articles for this subproject
Main Center: R832415
191 publications for this center
144 journal articles for this center
