Grantee Research Project Results
Final Report: Molecular Mechanisms
EPA Grant Number: R834514C003Subproject: this is subproject number 003 , established and managed by the Center Director under grant R834514
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Predictive Toxicology Center for Organotypic Cultures and Assessment of AOPs for Engineered Nanomaterials
Center Director: Faustman, Elaine
Title: Molecular Mechanisms
Investigators: Faustman, Elaine , Fenske, Richard , Griffith, William C. , Yost, Michael , Costa, Lucio G , Furlong, Clement , Thompson, Engelberta , Vigoren, Eric M. , Carr, Catherine J
Institution: University of Washington
EPA Project Officer: Callan, Richard
Project Period: September 25, 2008 through September 24, 2016
RFA: Children's Environmental Health and Disease Prevention Research Centers (with NIEHS) (2009) RFA Text | Recipients Lists
Research Category: Children's Health , Human Health
Objective:
- To investigate the direct effects of organophosphates (OPs) on neurite outgrowth, neuronal proliferation and viability in neurodevelopmental-stage specific in vitro models.
- To elucidate the impact of OP-induced oxidative stress and its effects on neuritogenesis and neurogenesis in neurodevelopmental-stage specific in vitro models.
- To examine developmental stage dependent impacts of chlorpyrifos (CP) on proliferation and differentiation gene expression pathways.
- To investigate whether OP exposure results in impairment of glial-neuronal interactions affecting the ability of astrocytes to promote neuritogenesis.
Work in Dr. Costa’s laboratory has focused on the effects of OPs on neuritogenesis and possible underlying mechanisms, thus encompassing Aims 1, 2, and 4. Work in Dr. Faustman’s lab encompasses Aims 1, 2, and 3.
Summary/Accomplishments (Outputs/Outcomes):
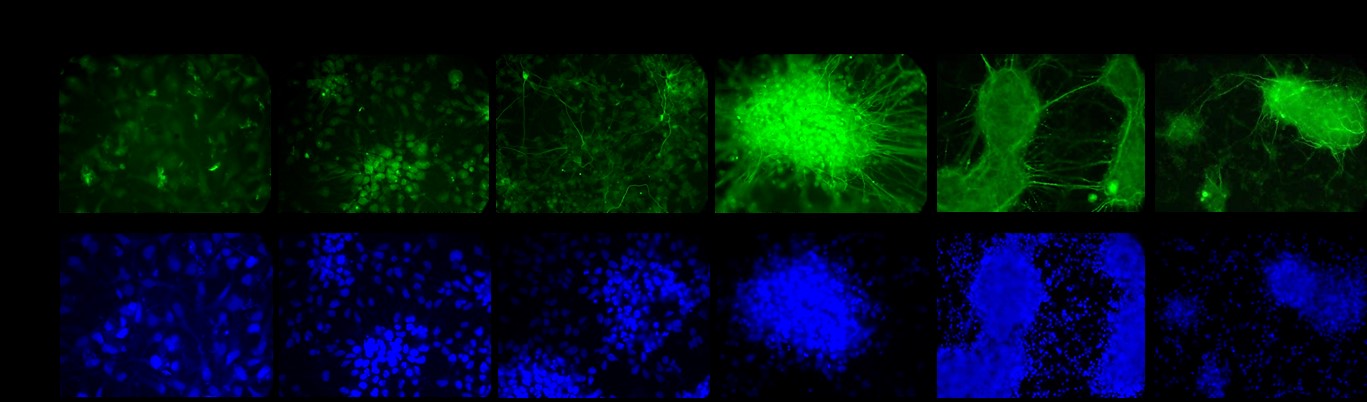
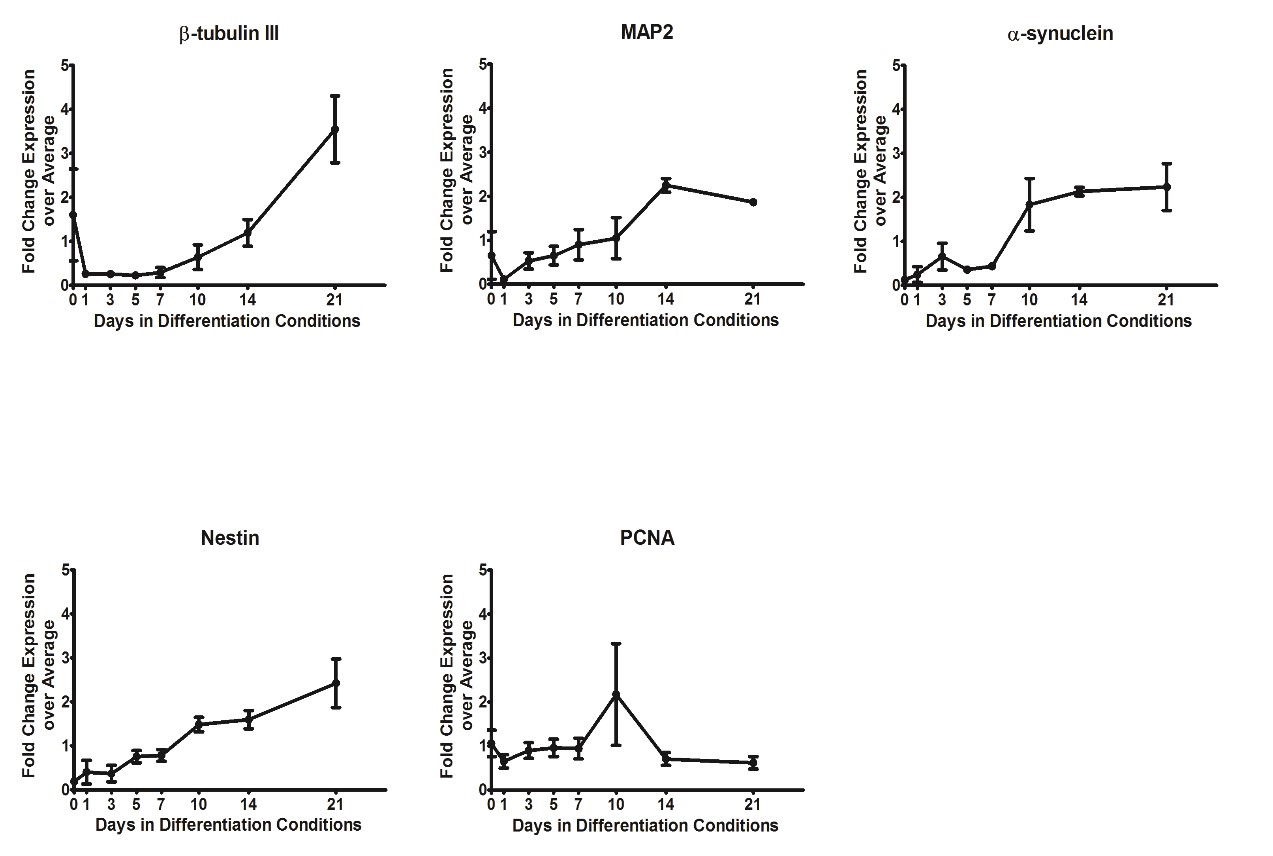
In addition, Dr. Faustman’s laboratory has characterized pathway dynamics throughout neuronal differentiation of the hNPC line that provides a particularly promising, scalable, and reproducible model for high-throughput and high-content neurodevelopmental toxicity screening. The laboratory cultured hNPCs up to 21 days in differentiation conditions and used Western blotting and immunofluorescence to measure changes in protein expression though time (Figures 3 and 4). Global gene expression dynamics were measured using Affymetrix Human gene 2.0 ST microarrays. Over time in differentiation conditions, hNPCs acquired morphological characteristics of mature neuronal networks and increased expression of neuronal differentiation markers, including beta tubulin III, MAP2 and synaptophysin. Significantly changed genes were organized according to temporal expression patterns using K-means clustering, revealing three phases of gene expression. Quantitative pathway analysis identified gene ontology (GO) terms enriched among genes expressed in each of these phases and created a quantitative summary or temporal pathway trends in vitro. GO terms enriched among genes significantly decreased over time are largely associated with proliferation and stem cell maintenance. GO terms enriched among genes with significantly increasing expression over time are dominated by key developmental processes, including neuronal differentiation, migration, and synaptogenesis. Enrichment of several GO terms associated with forebrain development indicates that these culture conditions promote differentiation towards a forebrain identity.
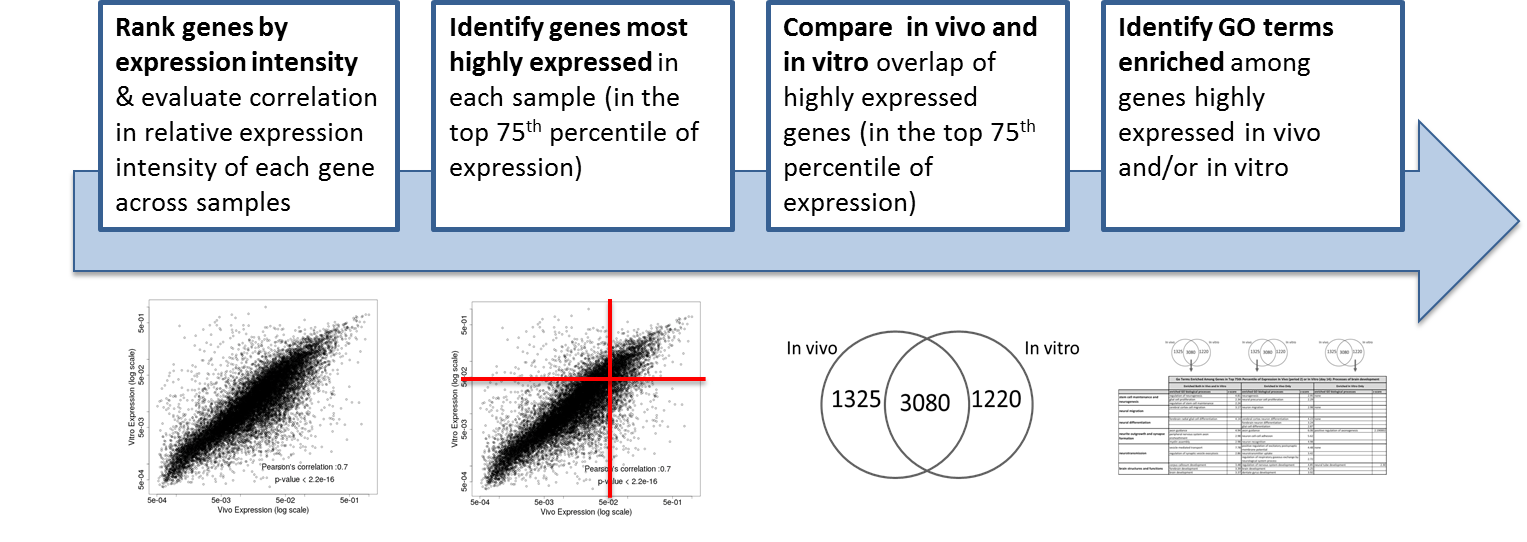
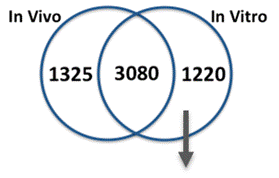
We compared gene expression in vitro with publicly available gene expression data from developing human brain tissue in vivo and found substantial concordance in relative gene expression intensity (Figure 5). Genes highly expressed in both samples were enriched for key processes of brain development, including proliferation, migration, differentiation, synapse formation, and neurotransmission (Table 1). Conversely, GO terms enriched among genes highly expressed only in vivo or only in vitro reveal important differences between systems. For example, genes highly expressed in vitro are enriched for more stress and apoptosis pathways. This analysis provides a timeline of progression through differentiation, facilitating identification of key phases of sensitivity in vitro. Key processes important for the identification of Adverse Outcome Pathways (AOPs) of proliferation, differentiation, and functional maturation matched in vivo patterns (Table 1). Given the heightened sensitivity of the brain to toxicant perturbation during critical windows of development, it is important that we understand which sensitive developmental pathways are captured in vitro and which are not so that in vitro assays can be interpreted appropriately. These observations of morphology, protein, and gene expression provide a timeline of progression through differentiation, facilitating identification of key phases of sensitivity. By anchoring in vitro dynamics to in vivo reference points, this work clarifies the extent to which fundamental processes of brain development are captured in our model.
| | 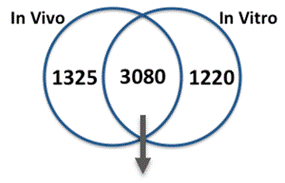 | 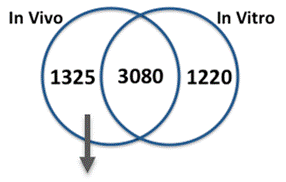 |  | |||||
| GO Terms Enriched Among Genes Above the 75th Percentile of Expression In Vivo (Period 2) and In Vitro (Day 14): Processes of Brain Development | ||||||||
| Enriched Both In Vivo and In Vitro | Enriched In Vivo Only | Enriched In Vitro Only | ||||||
| Enriched GO Biological Processes | Z-Score | Enriched GO Biological Processes | Z-Score | Enriched GO Biological Processes | Z-Score | |||
| Stem Cell Maintenance | Glial cell proliferation | 2.34 | Neural precursor cell proliferation | 2.29 | None | | ||
| Regulation of stem cell maintenance | 2.24 | | | | | |||
| | | | | | | |||
| Neurogenesis | Regulation of neurogenesis | 4.81 | Neurogenesis | 2.95 | None | | ||
| | | | | | | |||
| | | | | | | |||
| Neural Migration | Cerebral cortex cell migration | 3.17 | Neuron migration | 2.98 | None | | ||
| | | | | | | |||
| | | | | | | |||
| Neural Differentiation | Forebrain radial glial cell differentiation | 4.14 | Cerebral cortex neuron differentiation | 4.23 | None | | ||
| | | Forebrain neuron differentiation | 3.24 | | | |||
| | | Glial cell differentiation | 2.87 | | | |||
| Neurite Outgrowth and Synapse Formation | Axon guidance | 4.94 | Axon guidance | 6.06 | Positive regulation of axonogenesis | 2.19 | ||
| Peripheral nervous system axon ensheathment | 2.98 | Neuron cell-cell adhesion | 5.62 | | | |||
| Myelin assembly | 2.98 | Neuron recognition | 4.98 | | | |||
| Neurotransmission | Vesicle-mediated transport | 7.78 | Positive regulation of excitatory postsynaptic membrane potential | 4.48 | None | | ||
| Regulation of synaptic vesicle exocytosis | 2.86 | Neurotransmitter uptake | 3.42 | | | |||
| | | Regulation of respiratory gaseous exchange by neurological system process | 2.72 | | | |||
| Brain Structures and Functions | Corpus callosum development | 3.48 | Regulation of nervous system development | 4.85 | Neural tube development | 2.30 | ||
| Forebrain development | 3.39 | Brain development | 4.25 | | | |||
| Brain development | 3.37 | Dentate gyrus development | 3.92 | | | |||
Conclusions:
- Both DZ and DZO adversely affect astrocyte function, resulting in inhibited neurite outgrowth in hippocampal neurons. Inhibited outgrowth is associated with neurodegenerative diseases.
- DZ and DZO increase oxidative stress in astrocytes, and this in turn modulates astrocytic fibronectin, leading to impaired neurite outgrowth in hippocampal neurons.
- DZ and DZO significantly inhibited neurite outgrowth in hippocampal neurons at concentrations devoid of cyototoxicity. These effects appeared to be mediated by oxidative stress, as they were prevented by antioxidants (melatonin, N-t-butyl-alpha-phenylnitrone, and glutathione ethyl ester).
- Inhibition of neurite outgrowth was observed at concentrations below those required to inhibit the catalytic activity of acetylcholinesterase. DZ and DZO inhibit neurite outgrowth in hippocampal neurons by mechanisms involving oxidative stress, and these effects can be modulated by astrocytes and astrocyte-derived GSH. These responses are similar to those observed following OP exposure.
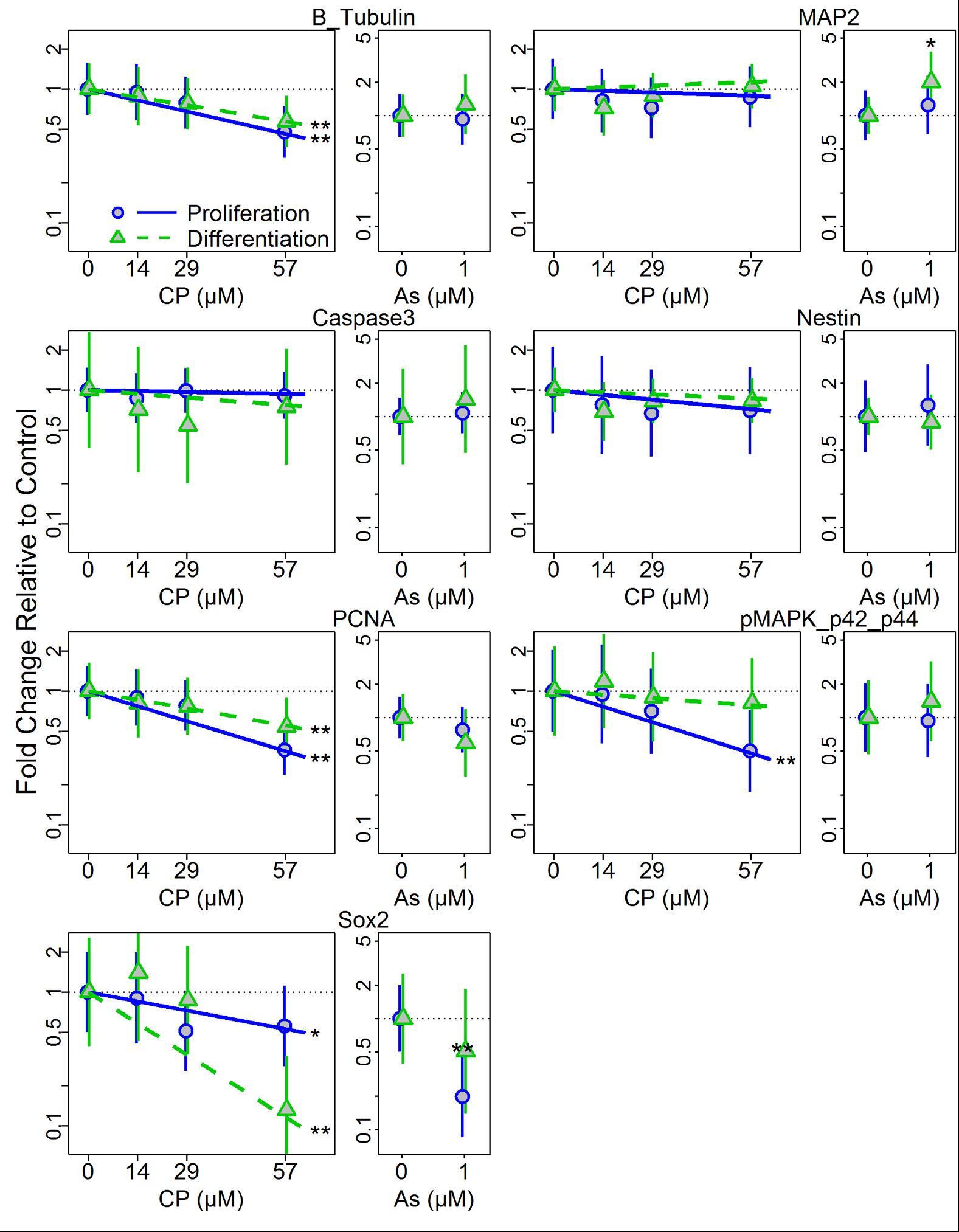
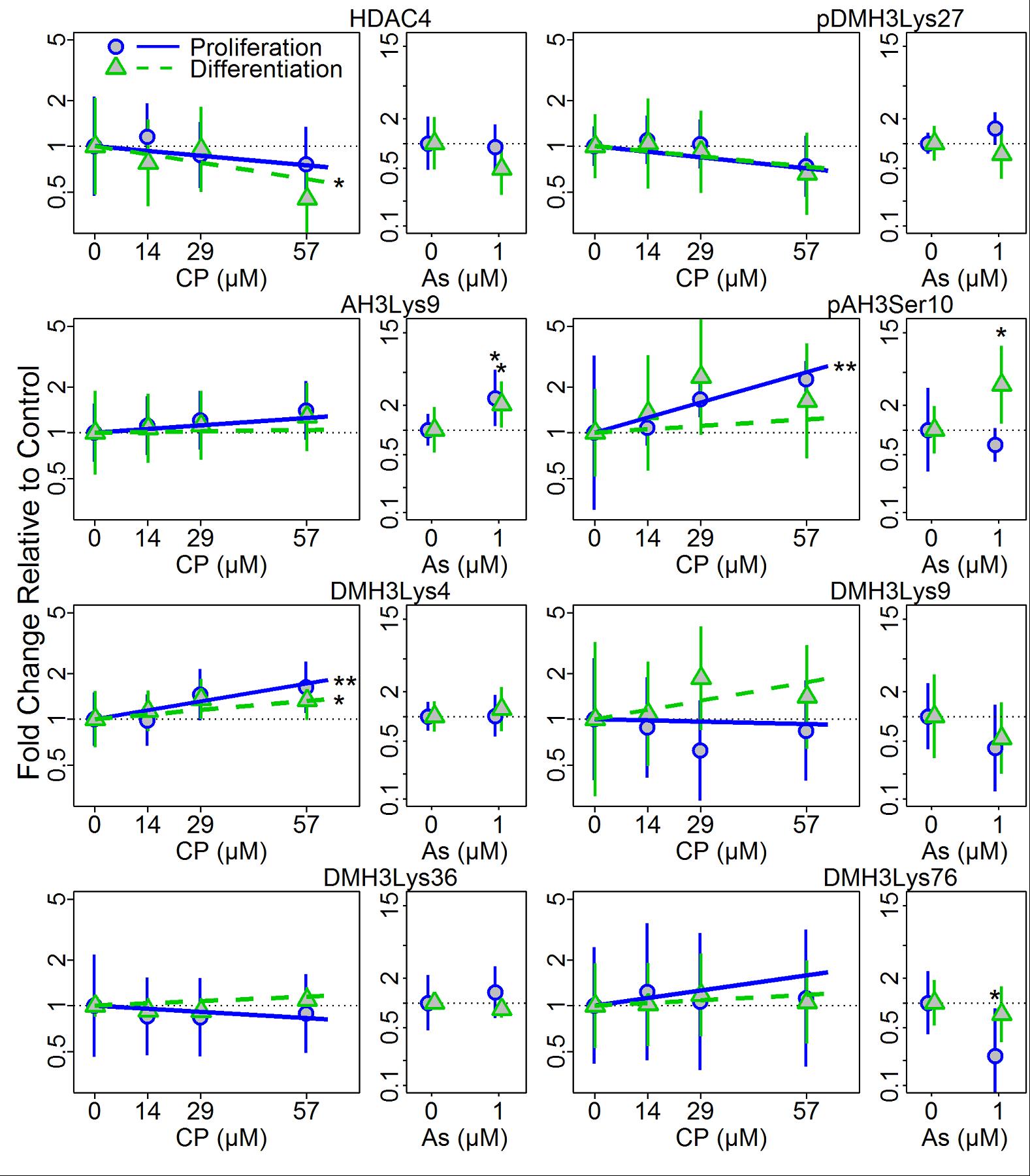
- Differentiation status (or developmental context) modifies the epigenetic effects of CP and As exposure.
- Characterization of neurodevelopmental toxicants using human neuroprogenitor cells provides a particularly promising, scalable, and reproducible model for high-throughput and high content neurodevelopmental toxicity screening.
- We have identified common mechanisms of actions for OP pesticides that have informed our analysis of adverse outcome pathways, which link OPs to adverse neurodevelopmental outcomes.
References:
- Giordano G, Afsharinejad Z, Guizzetti M, Vitalone A, Kavanagh T, Costa L. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicology and Applied Pharmacology 2007;219:181-189.
- Giordano G, Pizzuro D, VanDeMark K, Guizzetto M, Costa LG. Manganeze inhibits the ability of astrocytes to promote neuronal differentiation. Toxicology and Applied Pharmacology 2009;240:226-235.
- Guizzetti M, Pathak S, Giordano D, Costa L. Effect of organophosporus insecticides and their metabolites on astroglial cell proliferation. Toxicology 2005;215:182-190.
- Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. Journal of Biological Chemistry 2008;283:1884-1897.
- Guizzetti M, More NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia 2010;58:1395-1406.
- Pizzurro DM, Dao K, Costa LG. Diazinon and diazoxon impair the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons. Toxicology and Applied Pharmacology 2014a;274(3):372-382.
- Pizzurro DM, Dao K, Costa LG. Astrocytes protect against diazinon- and diazoxon-induced inhibition of neurite outgrowth by regulating neuronal glutathione. Toxicology 2014b;318:59-68.
Journal Articles on this Report : 16 Displayed | Download in RIS Format
| Other subproject views: | All 55 publications | 38 publications in selected types | All 16 journal articles |
|---|---|---|---|
| Other center views: | All 510 publications | 227 publications in selected types | All 178 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Costa LG, Pellacani C, Dao K, Kavanagh TJ, Roque PJ. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. NeuroToxicology 2015;48:68-76. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Harris S, Hermsen SA, Yu X, Hong SW, Faustman EM. Comparison of toxicogenomic responses to phthalate ester exposure in an organotypic testis co-culture model and responses observed in vivo. Reproductive Toxicology 2015;58:149-159. |
R834514 (Final) R834514C003 (2015) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2017) |
Exit Exit Exit |
|
|
Harris S, Wegner S, Hong SW, Faustman EM. Phthalate metabolism and kinetics in an in vitro model of testis development. Toxicology in Vitro 2016;32:123-131. |
R834514 (Final) R834514C003 (Final) R833772 (2009) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2016) R835738C004 (2017) |
Exit Exit Exit |
|
|
Harris S, Shubin SP, Wegner S, Van Ness K, Green F, Hong SW, Faustman EM. The presence of macrophages and inflammatory responses in an in vitro testicular co-culture model of male reproductive development enhance relevance to in vivo conditions. Toxicology In Vitro 2016;36:210-215. |
R834514 (Final) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2016) R835738C004 (2017) |
Exit Exit |
|
|
Kim HY, Wegner SH, Van Ness KP, Park JJ, Pacheco SE, Workman T, Hong S, Griffith W, Faustman EM. Differential epigenetic effects of chlorpyrifos and arsenic in proliferating and differentiating human neural progenitor cells. Reproductive Toxicology 2016;65:212-223. |
R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Moreira EG, Yu X., Robinson JF, Griffith W, Hong SW, Beyer RP, Bammler TK, Faustman EM. Toxicogenomic profiling in maternal and fetal rodent brains following gestational exposure to chlorpyrifos. Toxicology and Applied Pharmacology 2010;245(3):310-325. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (2007) |
Exit Exit Exit |
|
|
Pizzurro DM, Dao K, Costa LG. Astrocytes protect against diazinon- and diazoxon-induced inhibition of neurite outgrowth by regulating neuronal glutathione. Toxicology 2014;318:59-68. |
R834514C003 (Final) |
Exit Exit Exit |
|
|
Pizzurro DM, Dao K, Costa LG. Diazinon and diazoxon impair the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons. Toxicology and Applied Pharmacology 2014;274(3):372-382. |
R834514C003 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Guerrette Z, Yu X, Hong S, Faustman EM. A systems-based approach to investigate dose-and time-dependent methylmercury-induced gene expression response in C57BL/6 mouse embryos undergoing neurulation. Birth Defects Research. Part B: Developmental and Reproductive Toxicology 2010;89(3):188-200. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) |
Exit |
|
|
Robinson JF, Griffith WC, Yu X, Hong S, Kim E, Faustman EM. Methylmercury induced toxicogenomic response in C57 and SWV mouse embryos undergoing neural tube closure. Reproductive Toxicology 2010;30(2):284-291. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Yu X, Moreira EG, Hong S, Faustman EM. Arsenic-and cadmium-induced toxicogenomic response in mouse embryos undergoing neurulation. Toxicology and Applied Pharmacology 2011;250(2):117-129. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Theunissen PT, van Dartel DA, Pennings JL, Faustman EM, Piersma AH. Comparison of MeHg-induced toxicogenomic responses across in vivo and in vitro models used in developmental toxicology. Reproductive Toxicology 2011;32(2):180-188. |
R834514 (Final) R834514C003 (Final) |
Exit Exit Exit |
|
|
Wegner SH, Yu X, Pacheco Shubin S, Griffith WC, Faustman EM. Stage-specific signaling pathways during murine testis development and spermatogenesis: a pathway-based analysis to quantify developmental dynamics. Reproductive Toxicology 2015;51:31-39. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2017) |
Exit Exit Exit |
|
|
Wegner S, Yu X, Kim HY, Harris S, Griffith WC, Hong S, Faustman EM. Effect of dipentyl phthalate in 3-dimensional in vitro testis co-culture is attenuated by cyclooxygenase-2 inhibition. Journal of Toxicology and Environmental Health Sciences 2014;6(8):161-169. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit |
|
|
Yu X, Robinson JF, Sidhu JS, Hong S, Faustman EM. A system-based comparison of gene expression reveals alterations in oxidative stress, disruption of ubiquitin-proteasome system and altered cell cycle regulation after exposure to cadmium and ethylmercury in mouse embryonic fibroblast. Toxicological Sciences 2010;114(2):356-377. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (2007) |
Exit Exit |
|
|
Zhou C, Chen J, Zhang X, Costa LG, Guizzetti M. Prenatal ethanol exposure up-regulates the cholesterol transporters ATP-binding cassette A1 and G1 and reduces cholesterol levels in the developing rat brain. Alcohol and Alcoholism 2014;49(6):626-634. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
Supplemental Keywords:
RFA, Scientific Discipline, Health, INTERNATIONAL COOPERATION, ENVIRONMENTAL MANAGEMENT, Biochemistry, Environmental Monitoring, Children's Health, Environmental Policy, Biology, Risk Assessment, pesticide exposure, age-related differences, pesticides, children's vulnerablity, molecular research, biological markers, agricultural communityProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R834514 Predictive Toxicology Center for Organotypic Cultures and Assessment of AOPs for Engineered Nanomaterials Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R834514C001 Community-Based Participatory Research
R834514C002 Pesticide Exposure Pathways
R834514C003 Molecular Mechanisms
R834514C004 Genetic Susceptibility
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2015 Progress Report
- 2014
- 2013 Progress Report
- 2012
- 2011 Progress Report
- 2010
- 2009
- Original Abstract
16 journal articles for this subproject
Main Center: R834514
510 publications for this center
178 journal articles for this center