Grantee Research Project Results
2013 Progress Report: Center for Child Environmental Health Risks Research
EPA Grant Number: R834514Center: Predictive Toxicology Center for Organotypic Cultures and Assessment of AOPs for Engineered Nanomaterials
Center Director: Faustman, Elaine
Title: Center for Child Environmental Health Risks Research
Investigators: Faustman, Elaine , Fenske, Richard , Griffith, William C. , Yost, Michael , Costa, Lucio G , Furlong, Clement , Thompson, Engelberta , Vigoren, Eric M. , Karr, Catharine J.
Institution: University of Washington
EPA Project Officer: Callan, Richard
Project Period: September 25, 2009 through September 24, 2016
Project Period Covered by this Report: September 25, 2012 through September 24,2013
Project Amount: $5,417,075
RFA: Children's Environmental Health and Disease Prevention Research Centers (with NIEHS) (2009) RFA Text | Recipients Lists
Research Category: Children's Health , Human Health
Objective:
Since 1998, researchers of the University of Washington Center for Child Environmental Health Risks Research (the Center) have been using a multi-disciplinary research approach working in the lab, in the field, and in the community to understand the mechanisms that define childrens susceptibility to pesticides, identify the implications of this susceptibility for development and learning, and partner with our communities to translate our findings into risk communication, risk management, and prevention strategies. The Center is jointly funded by the National Institute of Environmental Health Sciences (NIEHS) and the Environmental Protection Agency (EPA).
The Center is administratively housed within the Institute for Risk Analysis and Risk Communication, also directed by Center Director Dr. Elaine M. Faustman, which is in the University of Washingtons School of Public Health. The Center includes partnerships with the Fred Hutchinson Cancer Research Center and the Yakima Valley community, located in the agricultural center of Washington State, to jointly sponsor a Community Based Participatory Research (CBPR) Project aimed at reducing childhood pesticide exposure.
All Center efforts are highly integrated with two field-based research projects, two laboratory-based research projects, three facility cores, an Administrative Core, and Faculty Development Investigator.
The specific objectives of the two field-based projects—the CBPR project and the pesticide exposure pathways research project—are to:
- Improve our understanding of critical pathways of potential pesticide exposure for children; and
- Apply culturally appropriate interventions to reduce childrens exposure to pesticides.
The specific objectives of the laboratory-based research projects—a molecular mechanisms research project and a genetic susceptibility research project—are to:
- Identify cellular, biochemical and molecular mechanisms that cause adverse developmental neurotoxicity of pesticides; and
- Identify susceptibility factors for developmental neurotoxicity of pesticides.
The three facility cores are: Biomarkers and Exposure Assessment (BEA); Biostatistics, Modeling and Risk Characterization (BMRC); and Community Outreach and Translation (COTC). The cores are designed to support the research objectives and to put the research into a child-specific risk assessment context. Thus, the specific objectives of the facility cores are to:
- Provide core support for the development and application of risk assessment methods, enabling basic research on pesticide toxicity and exposure to inform risk decisions to protect childrens health from pesticides; and
- Foster partnerships between academic researchers and the community in which information requested by the community and basic research deficiencies/gaps are translated into studies that address the needs of both.
The Administrative Core provides fiscal oversight, resource management, coordination, and integration of Center activities. The COTC shares membership with the Administrative Core and includes Dr. Catherine Karr, who since 2009 has served as the Centers Faculty Development Investigator, as well as a Pediatric Health Specialist. Center support and mentoring enable Dr. Karr to capitalize on the complementary and collaborative resources at the Center in order to navigate the transition to an independent investigator in this subject area.
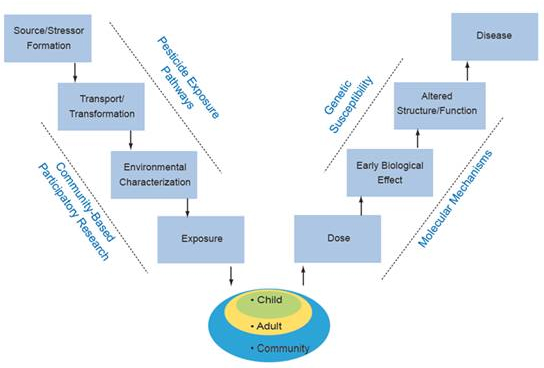
Work in the Center is organized around the Public Health paradigm V-diagram, which connects occurrence of disease in humans to the original source of the problem (see Figure 1). Along the pathway from source/stressor to disease, the diagram identifies intermediate processes (which may be subject to public health intervention) and conditions (which may be observable for public health monitoring and hypothesis testing). This paradigm is central to the overall organization and integration of the various components of the Center. Using Global Positioning System (GPS) tools to relate human activities and land use helps identify how organophosphate (OP) pesticide (stressor) application is transferred/transported to create OP environmental exposure conditions. The CBPR Project focuses on the multiple pathways of OP transfer to identify potential exposure and resulting dose in children. The biological sampling efforts help identify susceptibility factors and genetic mechanisms relating dose to early biological effect. Biostatistical analyses and outreach link these research components together.
Figure 1. The Public Health paradigm "V-diagram" frames and integrates the Center's research efforts.
Progress Summary:
Community Based Participatory Research Project
A key feature of the Center is the CBPR Project, which identifies the multiple potential pathways that may contribute to pesticide exposure in adults and children living in agricultural communities. Unlike many other studies of potential pesticide exposure, the CBPR investigators have used a group randomized trial design. Advantages of such an approach are the potential to reach large numbers of people, disseminate messages about behavior across a diverse population, change community norms about behavior, and integrate new behavioral practices into existing community structures and organizations. Part of the study design requires early involvement from the communities to guide us in planning intervention activities. The results of this research support the hypothesis that the take-home exposure pathway contributes to residential pesticide contamination in agricultural homes where young children are present.
The highlights of this past year include two Community Forums held in February 2013 in order to share information on the importance of donating specimens to biomedical research. The first was held in English for the Community Advisory Board, and the second was held in Spanish and was open to the community. These forums were a joint effort by Dr. Thompson with the CHC Center and Dr. Thompson's Community Based Participatory Research Project and NCI-funded Community Network Program Center (CNPC). The forums provided an opportunity to be transparent about the knowledge we hoped to gain from this study, and allowed us to gauge the audiences' understanding of our work. In the final phase of the study, dissemination tools will be designed with the feedback from these two forums in mind.
Pesticide Exposure Pathways Research Project
The Pesticide Exposure Pathways Research Project's overall aim is to develop a systematic understanding of residential pesticide exposure pathways arising from proximity to agricultural spraying in rural agricultural communities where children live. The proximity exposure pathway is due to transport of pesticides from applied fields to homes and other settings. The mechanisms of transport include spray drift, volatilization, re‐suspension and deposition of particles containing pesticides. The Pesticide Exposure Pathways Research Project studies both local pesticide application factors and human activity factors that contribute to childhood exposure to pesticides.
The highlights of this past year include air sampling to measure concentrations of airborne organophosphate pesticides (chlorpyrifos, azinphos-methyl) and oxon analogs at 20 participant homes. Chemical analysis using LC-MS-MS in the Environmental Health Laboratory was completed for ~150 samples. The data analysis for the initial results has been prepared for publication. Fieldworker observation and public land-use data were used to specify type of crop for proximal homes, emphasizing homes that were near tree fruit fields. This information will be used on the next stage of analysis for building exposure models.
These efforts address key aims of the current study by directly measuring exposures to OP pesticides inside and outside the participant homes and examining the potential contribution of the take-home and proximity pathways. PUF-PAS (polyurethane foam passive air sampling) disks have been developed and tested as a novel sampling device for studying pesticide exposures. PUF-PAS is highly effective for capturing airborne organophosphate pesticides while minimizing invasiveness to research participant families. Over 90% of the study participants that were approached participated in the air monitoring portion of the study, and only one passive sampling instrument had a failure. A paper has been submitted describing this new sampling method.
Our initial field results show that residences proximal (< 250 m) to fields experience higher monthly outdoor levels of chlorpyrifos and azinphos-methyl than non-proximal homes (> 250 m). Farmworker homes also experienced higher levels of outdoor pesticide concentrations, but this may be attributable to a relationship between increased proximity to tree fruit orchards and increased outdoor pesticide concentrations. Many farmworker homes were located in agriculturally dense areas. This is the first major study to validate a passive sampling device for OP pesticides and their oxon analogs. Passive sampling provides average exposures estimates over the course of several days and will dramatically enhance our ability to survey more participants and to collect samples over the course of an entire season. Both proximity and take-home routes contribute essentially independently to airborne OP pesticide exposures. This information will be a direct benefit for constructing exposure estimates for longer term epidemiological cohort studies in the future.
Molecular Mechanisms Research Project
One of the objectives of the Molecular Mechanisms research project is to understand how exposure to chemical and nonchemical stressors can impact childrens' neurological developmental processes. In order to prevent and predict developmental neurotoxicity, it is important to understand the who, what, where and when of chemical and non-chemical stressor biology and exposure. A key focus of this research project is to understand who is exposed, to which environmental chemicals, where the exposure is coming from and to identify biological impacts occurring across the life-course. For example, the Center investigates how an exposure early in development may differ significantly from impacts seen later when exposures occur at a different stage of development of life-course (toddler versus early school age children). Once we have characterized the exposures, we can use this information to make predictions about chemicals that could adversely impact neurodevelopment and then take action to prevent those exposures, especially in susceptible populations, such as children or pregnant women.
Our Center is particularly interested in pesticide exposure across the life-course. Pesticides can have adverse impacts on brain and nervous system development by altering the regulatory dynamics of normal central nervous system cell proliferation, differentiation and cell death, which can result in altered morphogenesis. These alterations are correlated with subsequent deficits in learning and development. A thorough understanding of mechanisms of toxicity at the molecular, cellular and organism level is therefore important as these mechanisms may define unique life stages that may be windows of susceptibility for many neurodevelopmental toxicants as well as for pesticides. The Molecular Mechanism Research Project evaluates these mechanistic processes across several pesticide classes in order to understand the commonality and uniqueness of pesticide toxicity across dose, life-course, cell type, and behavioral domain.
This research project brings together the expertise from different laboratories to focus on the relationship between pesticide‐induced alterations in cell proliferation and death during embryonic/postnatal development and postnatal alterations in behavior. These projects use rodent models and follow both in vivo and in vitro impacts. The laboratory of Dr. Elaine Faustman addresses the impacts of pesticides and model neurotoxicants on prenatal processes generating neurons, known as neurogenesis, and the laboratory of Dr. Lucio Costa evaluates the postnatal effects of pesticides on processes of generating non-neuronal glial cells, known as gliogenesis.
In the last several years, the Faustman lab initiated studies to explore new in vitro models for the evaluation of neurogenesis including assessment of proliferation and differentiation processes for in vivo comparison. The highlights of this year include an in vitro human neural progenitor cell model that was established to study adverse effects of the OP pesticide chlorpyrifos (CP) during neuronal proliferation and differentiation. Using confocal imaging to characterize cell-by-cell expression of markers of differentiation and proliferations will help illuminate developmental processes occurring in these cell cultures through both normal development and when perturbed by pesticide exposure. Understanding these dynamics will help improve and stabilize culture conditions to more accurately reflect in vivo developmental processes. Towards this goal, the researchers have characterized protein expression and this expression has been compared to the Allen Developing Mouse Brain Atlas reports on gene expression. The researchers have included not only in vitro human neuronal progenitor cells with in vivo murine brain development but also have added a comparison with their in vitro murine Central Nervous Systems micromass cell cultures.
Another highlight of recent work are the results of our studies suggesting that epigenetic factors play a role in the neurodevelopmental effects associated with CP exposure. For example, toxicogenomic profiling of CP exposed fetal and maternal mouse brains showed significant alteration to chromatin modifications. Exposure to CP during critical windows of susceptibility has been reported to alter behavior and development of the central nervous system by interfering with the regulatory pathways that control the production and selective cell loss of neurons. These results showing differential alterations of epigenetic markers during proliferation and differentiation can offer cell stage specific mechanistic information to help researchers tease out neurodevelopmental effects of the organophosphate pesticide CP.
The focus of Dr. Costas' project is the investigation of glial-neuronal interactions in neuronal differentiation. Work in Dr. Costas' laboratory has focused on the OP pesticide diazinon (DZ) and its active metabolite diazoxon (DZO). As both have been shown to induce oxidative stress in neurons, and based on previous studies with manganese, it was hypothesized that these OPs may induce oxidative stress also in astrocytes, and that this would impair the ability of these cells to induce normal neuronal differentiation, which is necessary for healthy neurological development. In our recent work, both DZ and DZO induced oxidative stress as assessed by measuring levels of reactive oxygen species (ROS) in rat cortical astrocytes. In separate experiments, the ability of OPs to directly affect neuritogenesis was also investigated. In this experimental protocol, neurons were cultured alone, in the presence of astrocyte-conditioned serum, but not in co-culture with astrocytes. Preliminary experiments indicate that when co-incubated with neurons, astrocytes are capable of increasing the levels of the protective antioxidant, glutathione (GSH). The increased GSH levels in neurons would thus explain their increased resistance to OPs. This was further confirmed by raising GSH levels in neurons with the use of GSH ethyl ester.
The complementary objectives of the Faustman and Costa laboratories have contributed to the advancement of our understanding of the potential for, and magnitude of, impacts of pesticides on neurogenesis and gliogenesis. In both of these essential neurodevelopmental pathways, the balance between initial proliferation and subsequent specific differentiation is integral for proper neurodevelopment. The integration of the Faustman and Costa laboratories allows us to better understand the molecular mechanisms that can cause adverse neurological development in children, following pesticide exposure. Our investigation of critical molecular pathways facilitating proliferation and toxicant response allow us to better predict neurological toxicity and increase our understanding about the importance of timing of exposure. The results from this project can be used to identify particularly vulnerable or sensitive populations and can be translated into information relevant for establishing conditions promoting environmental and public health safety.
Genetic Susceptibility Research Project
Activities of the Genetic Susceptibility Research Project have contributed to a greater understanding of the role of gene-environment interactions that define childrens' susceptibility to OP pesticides. This project interacts with many of the lab and field-based CHC projects as well as with many external collaborators. In particular, this project interacts with the Centers for Disease Control and Prevention (CDC), Environmental Protection Agency (USEPA) Region 10, Agency for Toxic Substances and Disease Registry (ATSDR), the University of California Berkeley Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), Washington State Department of Health, Washington State Pesticide Incident and Reporting Panel (PIRT), other U.S. government agencies, the United Kingdom (UK) Committee on Toxicity and several members of the UK Parliament.
The overall goal of the Genetic Susceptibility Research Project is to develop specific biomarkers of exposure to OP compounds and to use these biomarkers to explore gene-environment interactions related to genetic variability in the paraoxonase (PON1) gene, particularly with respect to OP exposures that occur during early development. The results of this project have helped create new more sensitive techniques for assessing pesticide exposure and greatly contributed to our understanding of how genetic differences in pesticide metabolism pathways impact adverse health responses across the life-course.
This project is developing many new techniques for the detection of adducts in blood samples that are associated with specific pesticide exposures. Highlights of the past year include experiments demonstrating that a single step immunomagnetic bead protocol will purify butyrylcholinesterase (BuChE) from plasma and acyl peptide hydrolase (APH) from red cell extracts. The OP adducts to these proteins have been identified. The serine adduct from the active metabolite of tri-cresyl phosphates was found to undergo a unique aging reaction. In vitro inhibition of BuChE with the active metabolite of TCP, cyclic saligenin cresyl phosphate (CBDP) generated both a cresyl phosphoserine and an aged phosphoserine. However, in samples from exposed individuals, only the fully aged phosphoserine modification was observed. More recent experiments have shown that acetyl cholinesterase (AChE) does not age in the same way and retains a cresyl group. We are currently developing methods to process samples for characterizing this adduct. Further, we have generated deuterated metabolite CBDP for generating in vitro heavy isotope labeled AChE for use as an internal standard. Characterization of the adducts on the active site serine of BuChE inhibited in vitro with chlorpyrifos oxon (CPO) showed the expected di-ethyl and aged mono-ethyl adducts while inhibition with azinphos methyl oxon generated the di-methyl and aged mono-ethyl serine adducts. An excellent correlation between our methods was developed. This was for the single step MS analysis of the active site serine of BuChE compared with the BuChE activity inhibition measurements made with thinning and non-spray season blood samples. These new techniques allow us quantify the effects of pesticide exposure with only one blood sample, rather than the requisite two samples needed by most current protocols.
One of the primary aims of this project in the previous funding period was to evaluate the effects of exposure to CPO during early postnatal development and to determine the role of the human PON1-Q192R polymorphism in modulating these effects, using multiple endpoints of OP toxicity. In the previous year, pilot studies were completed that determined steep chronic dose-response curves for inhibition of uBChE and AChE in pregnant and non-pregnant mice. Timed-matings and exposures of pregnant mice were completed. Mice with copulatory plugs were exposed transdermally to CPO daily. Dams were sacrificed, and fetal and maternal tissues were dissected for measurement of enzyme inhibition in different tissues. The PON1 status of the dam was found to clearly influence CPO toxicity to the fetus. RBC APH was found to be the most sensitive biomarker of CPO exposure, a particularly interesting finding given the potential importance of brain APH for cognition. A manuscript describing these data is in preparation. Because these same polymorphisms exist in human populations, understanding the changes in susceptibility of pregnant mice with varying PON1-status will help characterize human variability and identify similarly susceptible populations.
Additionally, a positive, linear correlation was found between plasma PON1 activity and AChE level of both brain and diaphragm in treated mice, indicating that plasma PON1 can serve as a susceptibility biomarker for DZO toxicity. Our research found that RBC APH is a useful biomarker for DZO exposure because of a good correlation between APH inhibition and AChE inhibition in brain and diaphragm. A manuscript describing these findings is now in preparation.
Biomarkers and Exposure Assessment Facility Core
The Biomarkers and Exposure Assessment facility core has focused on samples collected in the field. This core has coordinated their analysis for chemicals and other biomarkers of exposure and effect as well as characterized genotype and phenotype. A strong emphasis has been placed on providing analytical support for field samples collected by the CBPR Project and the Pesticide Exposure Pathways Research Project. The Core stores the results of the analyses and assists investigators in their interpretation. The Core also has provided support to the Genetic Susceptibility Research Project through the identification and development of relevant biomarkers.
Most recently, samples from the CBPR study were collected earlier in the year than in prior years to capture the time of CP pesticide use. The samples collected in the early spring that had not been collected in previous studies has allowed us to capture exposure to different types of OP pesticides, mainly CP, that are applied during this time. Samples also were collected during the early spring, late spring-early summer (to coincide with the timing of previous sample collections), and again in the off season during winter. Because children were older in our most recent field visit, it was possible to collect whole blood from the children so that we can examine a wider variety of biomarkers. Based on our results from our previous studies, we have expanded the list of biomarkers that are being studied.
The Core helps investigators develop new biomarkers, ranging from indicators of exposure to early markers of potential disease. The expanded analyses of OP parent compounds since 2009 includes selected oxons of OPs since these have been detected in environmental samples and are much more toxic than the parent compounds.
The Core collaborates closely with the UW Environmental Health Laboratory and Trace Organic Analytical Center and other laboratories to carry out these analyses. The Core continues to monitor and maintain the Center's biorepository and associated IRB applications and requests. Additional funding has been obtained from the National Childrens Study (NCS) to analyze samples in the biorepository collected in past Center studies. The results of these additional studies will be used to inform the Main NCS study as to best practices for the collection, storage, and analysis of field collected samples within a large longitudinal study. The Center has examined an expanded list of pyrethroids, neonicotinoids and fungicides. During the past year, this Core has coordinated analyses on field collected house and vehicle dust samples. Specifically, we characterized the occupational farmworker (FW) vs non-farmworker (NFW) and seasonal profiles for 16 organophosphate pesticides (OPs), 71 other pesticides, 22 metals, 18 phthalates and 50 molds. The analyses on molds in dust coordinated by the Core are reported in a published manuscript and represent 50 house dust samples from 24 households. The study compares pyrosequencing and the ERMI methodologies for characterizing molds by relative moldiness, a metric associated with an increase of childhood asthma. The paper illustrates how the two methods complement each other in measuring household mold and characterizing exposure. The data from these studies are being analyzed further to characterize how mold changes with agricultural season and household flooring type.
The Core has been working with the UW Environmental Health Laboratory to develop protocols for cortisol analysis in hair. A total of 54 hair samples were collected and have been analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS) for cortisol. Further protocol development is in progress for the cortisol analysis as well as an additional metal analysis. The Core also has completed saliva cortisol analysis as part of the stress study.
The Core also has developed protocols for biospecimen real-time analysis for informing the Main NCS study. The goal of this project was to identify and optimize state-of-the-art methods to analyze metabolic responses to environmental exposure using the newest omics field, metabolomics, which may provide a powerful way to search for and identify biomarkers for disease and exposures.
Biostatistics, Modeling and Risk Characterization Facility Core
The Core provides innovative statistical, modeling and risk assessment capabilities in order to interpret results of the Center's research. This core has used high-dimension, innovative statistical models for exposure analysis that allow for successful interpretation and analysis of complex cohort studies. Collaboration with investigators on statistical designs and analysis is the start of an integrated process whereby this Core has improved the initial design and construct of studies to increase their power to detect statistically significant results. Some of the challenges the Core has taken into account when performing analyses include field samples with frequent values below quantification limits, missing data, multiple data collection protocols, combinations of random and fixed effects, repeated measures, and multiple spatial and temporal scales.
Analysis of exposure data from the CBPR Projects' previous cohort and the current Pesticide Exposure Pathways Research Project has focused on using Bayesian Markov chain Monte Carlo methods using multivariate distributions in hierarchical models to understand the relationship between child and adult exposures from the take-home pathway for orchard workers, trends in exposures across agricultural seasons, differences between exposures of farmworker and non-farmworker household exposures, and sources of variability.
The Core has been deeply involved in the design of the field sampling to collect information on the cohort identified in previous Center studies. The Center has extended the time period for characterizing exposures by going into the field earlier than previous studies to include the early spring when different OP pesticides are used, in particular chlorpyrifos (CP). In the previous studies, the first season we collected was the thinning season in spring when dimethyl OP pesticides such as azinphos-methyl (AZ) are primarily used. By including the early spring, we will gain a more complete understanding of the episodic OP exposures of orchard workers and their children in the Yakima Valley.
At present, we have data on concentrations of OPs and other contaminants in dust. In the current study, we collected dust from two different days in each season, the first and last days on which we collect urine, in contrast to previous studies where we collected dust on a single day each season. We also are using a less costly method for collecting the dust using a hand-held vacuum. Our initial analyses show that while we collect less dust with the hand-held vacuum, the amounts of dust and concentrations of OPs in the dust provide consistent samples with correlations of 0.7 to 0.8 between the two days within a season and correlations of 0.4 to 0.5 between seasons. The correlations between seasons are similar to the correlations for OPs we saw in our previous Center studies when we collected dust on a single day in each season using a more expensive high powered vacuum. We also have data on additional pesticides and contaminants in the dust that we are in the process of analyzing.
In the current Center studies, researchers also are characterizing stress exposure through the analysis of the stress hormone, cortisol in the blood, saliva and hair of a subset of women. These analyses will help to determine the exposure to environmental and psychosocial stresses in farmworkers and non-farmworkers. We also have used this study to optimize stress assessment protocols through the integration of cortisol biomarker samples with stress questionnaire data. The Core has developed a biokinetic model of cortisol in order to relate the available cortisol biomarkers to estimated peak and average levels of cortisol. The biokinetic model of cortisol was developed based upon literature values and has been tested using blood and saliva samples from the current study. The biokinetic model has performed well, the original publication is already available and the results of our biomarker sample integration have been submitted for publication. Hair cortisol will be added once the sample analysis is complete.
The Core provides an integrated analysis of the results from the multiple studies in the Center. This integration is important for the proper interpretation of the results. In the Center, the studies collect multiple samples at multiple times and seasons from the same set of households, and then multiple analyses are performed on each sample. For the proper interpretation, it is important to have integrated analyses to describe the multivariate joint distribution of these results because all of the characteristics of the data impact the interpretation of individual variables and the relationship between the variables. The integration has allowed us to consider how genotypes for metabolizing enzymes alter the dose-response relationship between cholinesterase inhibition and exposure to OP pesticides.
Community Outreach and Translation Facility Core
The COTC is positioned within the Center to overcome the barriers involved in sharing, interpreting, translating, documenting and archiving data and information in an interdisciplinary and true collaborative research setting. The goal of COTC is to develop, implement and evaluate strategies to translate and apply the scientific findings of the Center into information for the public, policy makers and clinical professionals to use to protect the health of children. The COTC shares membership with the Administrative Core and includes the Centers Faculty Development Investigator/Pediatric Health Specialist, Dr. Karr.
During the reporting period, COTC members have been involved in a variety of exciting outreach and translational activities, on the local, the national, and the international level. Examples include coordinating journal club sessions, hosting an annual regional conference, implementing a webinar series, and developing outreach materials. In the coming year, the team plans also to develop a continuing education course. Because a major aim of the COTC is to engage the Pediatric Health Specialist in clinical translation and needs assessment, Dr. Karr's efforts related to this aim are reported here, and her other pediatric educational activities and pursuits related to her position as Faculty Development Investigator are reported in the Faculty Development Investigator summary.
A key aim of the COTC is the dissemination of research findings and policy implications to not only the broader research community but also government agencies, regulatory bodies, decision makers, the interested public and other stakeholder groups. During the reported period, COTC members were involved in a variety of exciting outreach and translational activities on both the local and national level. In January 2013, Drs. Karr and Faustman attended the Symposium on Cumulative Impacts and Childrens Health. This event was hosted by UC Berkeley and provided updates on scientific findings related to childrens health. Drs. Karr and Faustman assisted with planning this event, which was co-sponsored by the UW CHC and UW PEHSU. Dr. Faustman's presentation entitled "Integrating Temporal and Systems Based Models for Detecting Cumulative Impacts: Lessons from the Pacific Northwest Childrens Health Research Center" discussed models for detecting cumulative health impacts in children.
Another exciting highlight was a collaboration with UW Pediatric Environmental Health Specialty Unit (directed by Dr. Karr). The Center organized and implemented a second annual half-day conference highlighting important contributions to childrens' environmental health from academic or public health agency leaders in the Pacific Northwest. The conference took place on February 26, 2013. The topics covered during this year's conference included effects of wood smoke on children, obesity and childrens health. We were also pleased to be able to present updates on our Centers Return of Results to the community in Eastern Washington. This 2nd annual conference was well attended, drawing over 75 participants from academia, government agencies, clinics, students and the interested public. Feedback for the conference was very positive. The enthusiasm of the audience continues to encourage us to host these annual research conferences, and we anticipate next year's conference to continue this trend.
There are several ways in which Dr. Karr, as the Pediatric Health Specialist, translates Center activities and scientific findings to related health professionals. Primarily, this occurs through educational material in the forms of local presentations, Grand Rounds, participation in public health advisory panels, review article development for clinician and public health professional audiences, monthly journal club, etc. Dr. Karr also participates in review articles, policy development, technical reporting for public health professionals and pediatric clinical providers. Dr. Karr was asked to serve as co-chair for the case management workgroup for the new CDC grant to the Washington State Department of Health for the Washington State Healthy Homes Initiative. In doing so, Dr. Karr oversaw the group that is focused on developing and consolidating case management protocols for children suffering from home-related illnesses such as lead poisoning and asthma.
Future Activities:
>The highlights above illustrate the high volume and quality of the Center's efforts aiming at reducing the adverse effects of environmental pesticide exposures in children. The Center continues to work to understand the mechanisms that define children's susceptibility to pesticides, identify the implications of this susceptibility for health impacts on development and learning, and partner with our communities to translate our findings into risk communication, risk management and prevention strategies. Center researchers continue to work in the lab, in the field, and in the community to bring a unique and successful approach to the study of children's environmental health.
Journal Articles: 178 Displayed | Download in RIS Format
| Other center views: | All 510 publications | 227 publications in selected types | All 178 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Armstrong JL, Fenske RA, Yost MG, Galvin K, Tchong-French M, Yu J. Presence of organophosphorus pesticide oxygen analogs in air samples. Atmospheric Environment 2013;66:145-150. |
R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit |
|
|
Armstrong JL, Fenske RA, Yost MG, Tchong-French M, Yu J. Comparison of polyurethane foam and XAD-2 sampling matrices to measure airborne organophosphorus pesticides and their oxygen analogs in an agricultural community. Chemosphere 2013:92(4):451-457. |
R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit Exit |
|
|
Armstrong JL, Fitzpatrick CF, Loftus CT, Yost MG, Tchong-French M, Karr CJ. Development of a unique multi-contaminant air sampling device for a childhood asthma cohort in an agricultural environment. Environmental Science: Processes & Impacts 2013;15(9):1760-1767. |
R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit |
|
|
Armstrong JL, Dills RL, Yu J, Yost MG, and Fenske RA. A sensitive LC-MS/MS method for measurement of organophosphorus pesticides and their oxygen analogs in air sampling matrices. Journal of Environmental Science and Health, Part B 2014;49(2):102-108. |
R834514 (Final) R834514C002 (Final) |
Exit |
|
|
Armstrong JL, Yost MG, Fenske RA. Development of a passive air sampler to measure airborne organophosphorus pesticides and oxygen analogs in an agricultural community. Chemosphere 2014;111:135-143. |
R834514 (Final) R834514C002 (Final) |
Exit Exit Exit |
|
|
Asselbergs FW, Guo Y, van Iperen EP, Sivapalaratnam S, Tragante V, Lanktree MB, Lange LA, Almoguera B, Appelman YE, Barnard J, Baumert J, Beitelshees AL, Bhangale TR, Chen YD, Gaunt TR, Gong Y, Hopewell JC, Johnson T, Kleber ME, Langaee TY, Li M, Li YR, Liu K, McDonough CW, Meijs MF, Middelberg RP, Musunuru K, Nelson CP, O'Connell JR, Padmanabhan S, Pankow JS, Pankratz N, Rafelt S, Rajagopalan R, Romaine SP, Schork NJ, Shaffer J, Shen H, Smith EN, Tischfield SE, van der Most PJ, van Vliet-Ostaptchouk JV, Verweij N, Volcik KA, Zhang L, Bailey KR, Bailey KM, Bauer F, Boer JM, Braund PS, Burt A, Burton PR, Buxbaum SG, Chen W, Cooper-Dehoff RM, Cupples LA, deJong JS, Delles C, Duggan D, Fornage M, Furlong CE, Glazer N, Gums JG, Hastie C, Holmes MV, Illig T, Kirkland SA, Kivimaki M, Klein R, Klein BE, Kooperberg C, Kottke-Marchant K, Kumari M, LaCroix AZ, Mallela L, Murugesan G, Ordovas J, Ouwehand WH, Post WS, Saxena R, Scharnagl H, Schreiner PJ, Shah T, Shields DC, Shimbo D, Srinivasan SR, Stolk RP, Swerdlow DI, Taylor HA Jr, Topol EJ, Toskala E, van Pelt JL, van Setten J, Yusuf S, Whittaker JC, Zwinderman AH; LifeLines Cohort Study, Anand SS, Balmforth AJ, Berenson GS, Bezzina CR, Boehm BO, Boerwinkle E, Casas JP, Caulfield MJ, Clarke R, Connell JM, Cruickshanks KJ, Davidson KW, Day IN, de Bakker PI, Doevendans PA, Dominiczak AF, Hall AS, Hartman CA, Hengstenberg C, Hillege HL, Hofker MH, Humphries SE, Jarvik GP, Johnson JA, Kaess BM, Kathiresan S, Koenig W, Lawlor DA, März W, Melander O, Mitchell BD, Montgomery GW, Munroe PB, Murray SS, Newhouse SJ, Onland-Moret NC, Poulter N, Psaty B, Redline S, Rich SS, Rotter JI, Schunkert H, Sever P, Shuldiner AR, Silverstein RL, Stanton A, Thorand B, Trip MD, Tsai MY, van der Harst P, van der Schoot E, van der Schouw YT, Verschuren WM, Watkins H, Wilde AA, Wolffenbuttel BH, Whitfield JB, Hovingh GK, Ballantyne CM, Wijmenga C, Reilly MP, Martin NG, Wilson JG, Rader DJ, Samani NJ, Reiner AP, Hegele RA, Kastelein JJ, Hingorani AD, Talmud PJ, Hakonarson H, Elbers CC, Keating BJ, Drenos F. Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. American Journal of Human Genetics 2012;91(5):823-838. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Asselbergs, F.W., Y. Guo, E.P. van Iperen, S. Sivapalaratnam, V. et al. Large-Scale Gene-Centric Meta-analysis across 32 Studies Identifies Multiple Lipid Loci. Am J Hum Genet, 2012. 91(5):p. 823-38. |
R834514C004 (Final) R834798C005 (Final) |
not available |
|
|
Baker PE, Cole TB, Cartwright M, Suzuki SM, Thummel KE, Lin YS, Co AL, Rettie AE, Kim JH, Furlong CE. Identifying safer anti-wear triaryl phosphate additives for jet engine lubricants. Chemico-Biological Interactions 2013;203(1):257-264. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit Exit |
|
|
Bal-Price AK, Coecke S, Costa L, Crofton KM, Fritsche E, Goldberg A, Grandjean P, Lein PJ, Li A, Lucchini R, Mundy WR, Padilla S, Persico AM, Seiler AE, Kreysa J. Advancing the science of developmental neurotoxicity (DNT): testing for better safety evaluation. ALTEX 2012;29(2):202-215. |
R834514 (2013) |
Exit Exit |
|
|
Balk SJ, Council on Environmental Health, Section on Dermatology. Technical report—ultraviolet radiation: a hazard to children and adolescents. Pediatrics 2011;127(3):e791-e817. |
R834514 (2011) R834514 (2012) R834514 (Final) |
Exit Exit |
|
|
Below JE, Earl DL, Shively KM, McMillin MJ, Smith JD, Turner EH, Stephan MJ, Al-Gazali LI, Hertecant JL, Chitayat D, Unger S, Cohn DH, Krakow D, Swanson JM, Faustman EM, Shendure J, Nickerson DA, Bamshad MJ, University of Washington Center for Mendelian Genomics. Whole-genome analysis reveals that mutations in inositol polyphosphate phosphatase-like 1 cause opsismodysplasia. American Journal of Human Genetics 2013;92(1):137-143. |
R834514 (2013) |
Exit Exit |
|
|
Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Luscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. The Journal of Clinical Investigation 2011;121(7):2693-2708. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Buka I, Osornio-Vargas A, Karr C. Melamine food contamination: relevance to Canadian children. Paediatrics and Child Health 2009;14(4):222-224. |
R834514 (2012) R834514 (2013) R834514 (Final) |
|
|
|
Chen J, Zhang XL, Kusumo H, Costa LG, Guizzetti M. 2013. Cholesterol efflux is differentially regulated in neurons and astrocytes:Implications for brain cholesterol homeostasis. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 1831(2):263-275.PMC3534809. |
R834514 (2013) |
Exit |
|
|
Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environmental Health Perspectives 2010;118(2):284-290. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
|
|
|
Cole TB, Jansen K, Park S, Li W-F, Furlong CE, Costa LG. The toxicity of mixtures of specific organophosphate compounds is modulated by paraoxonase 1 status. Advances in Experimental Medicine and Biology 2010;660:47-60. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514C004 (Final) R831709 (2007) R831709 (Final) |
Exit Exit |
|
|
Cole TB, Beyer RP, Bammler TK, Park SS, Farin FM, Costa LG, Furlong CE. Repeated developmental exposure of mice to chlorpyrifos oxon is associated with paraoxonase 1 (PON1)-modulated effects on cerebellar gene expression. Toxicological Sciences 2011;123(1):155-169. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Cole TB, Giordano G, Co AL, Mohar I, Kavanagh TJ, Costa LG. Behavioral characterization of GCLM-knockout mice, a model for enhanced susceptibility to oxidative stress. Journal of Toxicology 2011;2011:157687 (7 pp.). |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Cole TB, Fisher JC, Burbacher TM, Costa LG, Furlong CE. Neurobehavioral assessment of mice following repeated postnatal exposure to chlorpyrifos-oxon. Neurotoxicology and Teratology. 2012;34(3):311-22. |
R834514 (Final) R834514C004 (Final) R834693 (2015) |
Exit Exit Exit |
|
|
Cole TB, Fisher JC, Burbacher TM, Costa LG, Furlong CE. Neurobehavioral assessment of mice following repeated postnatal exposure to chlorpyrifos-oxon. Neurotoxicology and Teratology 2012;34(3):311-322. |
R834514 (2011) R834514 (2012) R834514 (2013) R834693 (Final) |
Exit Exit Exit |
|
|
Committee on Environmental Health, Committee on Infectious Diseases, Rogan WJ, Brady MT. Drinking water from private wells and risks to children. Pediatrics 2009;123(6):1599-1605. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Committee on Environmental Health, Tester JM. The built environment: designing communities to promote physical activity in children. Pediatrics 2009;123(6):1591-1598. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Committee on Environmental Health, Committee on Substance Abuse, Committee on Adolescence, Committee on Native American Child Health. Tobacco use: a pediatric disease. Pediatrics 2009;124(5):1474-1484. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Coronado GD, Vigoren EM, Griffith WC, Faustman EM, Thompson B. Organophosphate pesticide exposure among pome and non-pome farmworkers:a subgroup analysis of a community randomized trial. Journal of Occupational and Environmental Medicine 2009;51(4):500-509. |
R834514 (2011) R834514 (Final) R834514C001 (Final) R831709 (2007) R831709 (Final) R832734 (Final) |
Exit |
|
|
Coronado GD, Griffith WC, Vigoren EM, Faustman EM, Thompson B. Where's the dust? Characterizing locations of azinphos-methyl residues in house and vehicle dust among farmworkers with young children. Journal of Occupational and Environmental Hygiene 2010;7(12):663-671. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C001 (Final) R831709 (Final) |
Exit |
|
|
Coronado GD, Holte S, Vigoren E, Griffith WC, Faustman E, Thompson B. Organophosphate pesticide exposure and residential proximity to nearby fields:evidence for the drift pathway. Journal of Occupational and Environmental Medicine 2011;53(8):884-891. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C001 (Final) R831709 (Final) |
Exit |
|
|
Coronado GD, Holte SE, Vigoren EM, Griffith WC, Barr DB, Faustman EM, Thompson B. Do workplace and home protective practices protect farm workers? Findings from the "For Healthy Kids" study. Journal of Occupational and Environmental Medicine 2012;54(9):1163-1169. |
R834514 (2013) R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Costa LG, Giordano G, Guizzetti M. In vitro models to study cell-cell interactions that influence developmental neurotoxicity. ALTEX 2010;27(Special Issue 1):303-308. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Costa LG, Giordano G, Furlong CE. Pharmacological and dietary modulators of paraxonase 1 (PON1) activity and expression: the hunt goes on. Biochemical Pharmacology 2011;81(3):337-344. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology 2013;307:115-122. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Costa LG. The birth and early years of INA, the International Neurotoxicology Association. Neurotoxicology 2013;36:89-103. |
R834514 (2013) |
Exit Exit |
|
|
Costa LG, Giordano G, Guizzetti M. Inhibition of cholinergic muscarinic signaling by ethanol: potential mechanism of developmental neurotoxicity and biological plausibility for the beneficial effects of choline supplementation. The International Journal of Alcohol and Drug Research 2013;2(3):17-25. |
R834514 (2013) |
Exit Exit |
|
|
Costa LG, Pellacani C, Dao K, Kavanagh TJ, Roque PJ. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. NeuroToxicology 2015;48:68-76. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Council on Environmental Health, Section on Dermatology. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics 2011;127(3):588-597. |
R834514 (2013) |
Exit Exit Exit |
|
|
Council on Environmental Health. Chemical-management policy: prioritizing children’s health. Pediatrics 2011;127(5):983-990. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Council on Environmental Health. Policy statement: pesticide exposure in children. Pediatrics 2012;130(6):e1757-e1763. |
R834514 (2012) |
Exit Exit |
|
|
Daniell WE, Van Tung L, Wallace RM, Havens DJ, Karr CJ, Bich Diep N, Croteau GA, Beaudet NJ, Duy Bao N. Childhood lead exposure from battery recyling in Vietnam. Biomed Research International 2015;2015:193715. |
R834514 (Final) |
Exit |
|
|
Furlong CE, Suzuki SM, Stevens RC, Marsillach J, Richter RJ, Jarvik GP, Checkoway H, Samii A, Costa LG, Griffith A, Roberts JW, Yearout D, Zabetian CP. Human PON1, a biomarker of risk of disease and exposure. Chemico-Biological Interactions 2010;187(1-3):355-361. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) R831709 (Final) |
Exit Exit Exit |
|
|
Furlong CE. Exposure to triaryl phosphates: metabolism and biomarkers of exposure. Journal of Biological Physics and Chemistry 2011;11(4):165-171. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Furlong CE, Marsillach J, Jarvik GP, Costa LG. Paraoxonases-1,-2 and-3: what are their functions? Chemico-Biological Interactions 2016;259(Pt B):51-62. |
R834514 (Final) |
Exit Exit Exit |
|
|
Garrick JM, Dao K, de Laat R, Elsworth J, Cole TB, Marsillach J, Furlong CE, Costa LG. Developmental expression of paraoxonase 2. Chemico-Biological Interactions 2016;259(Pt B):168-174. |
R834514 (Final) |
Exit Exit Exit |
|
|
Gibbs JL, Yost MG, Negrete M, Fenske RA. Passive sampling for indoor and outdoor exposures to chlorpyrifos, azinphos-methyl, and oxygen analogs in a rural agricultural community. Environmental Health Perspectives 2017;125(3):333-341. |
R834514 (Final) R834514C002 (Final) |
|
|
|
Giordano G, Guizzetti M, Dao K, Mattison HA, Costa LG. Ethanol impairs muscarinic receptor-induced neuritogenesis in rat hippocampal slices: role of astrocytes and extracellular matrix proteins. Biochemical Pharmacology 2011;82(11):1792-1799. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Giordano G, Cole TB, Furlong CE, Costa LG. Paraoxonase 2 (PON2) in the mouse central nervous system: a neuroprotective role? Toxicology and Applied Pharmacology 2011;256(3):369-378. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Giordano G, Costa LG. Developmental neurotoxicity: some old and new issues. ISRN Toxicology 2012;2012:814795. |
R834514 (2013) |
Exit Exit |
|
|
Giordano G, Kavanagh TJ, Faustman EM, White CC, Costa LG. Low-level domoic acid protects mouse cerebellar granule neurons from acute neurotoxicity: role of glutathione. Toxicological Sciences 2013;132(2):399-408. |
R834514 (2013) |
Exit Exit Exit |
|
|
Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, Costa LG. Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radical Biology and Medicine 2013;58:98-108. |
R834514 (2013) R834514C004 (Final) |
Exit Exit |
|
|
Griffith W, Curl CL, Fenske RA, Lu CA, Vigoren EM, Faustman EM. Organophosphate pesticide metabolite levels in pre-school children in an agricultural community:within-and between-child variability in a longitudinal study. Environmental Research 2011;111(6):751-756. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) R831709 (Final) |
Exit Exit Exit |
|
|
Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia 2010;58(12):1395-1406. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) R831709 (Final) |
Exit |
|
|
Guizzetti M, Moore NH, VanDeMark KL, Giordano G, Costa LG. Muscarinic receptor-activated signal transduction pathways involved in the neuritogenic effect of astrocytes in hippocampal neurons. European Journal of Pharmacology 2011;659(2-3):102-107. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Guo Y, Lanktree MB, Taylor KC, Hakonsarson H, Lange LA, Keating BJ, IBC 50K SNP Array BMI Consortium. Gene-centric meta-analyses of 108 912 individuals confirm known body mass index loci and reveal three novel signals. Human Molecular Genetics 2013;22(1):184-201. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Harris S, Hermsen SA, Yu X, Hong SW, Faustman EM. Comparison of toxicogenomic responses to phthalate ester exposure in an organotypic testis co-culture model and responses observed in vivo. Reproductive Toxicology 2015;58:149-159. |
R834514 (Final) R834514C003 (2015) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2017) |
Exit Exit Exit |
|
|
Harris S, Wegner S, Hong SW, Faustman EM. Phthalate metabolism and kinetics in an in vitro model of testis development. Toxicology in Vitro 2016;32:123-131. |
R834514 (Final) R834514C003 (Final) R833772 (2009) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2016) R835738C004 (2017) |
Exit Exit Exit |
|
|
Harris S, Shubin SP, Wegner S, Van Ness K, Green F, Hong SW, Faustman EM. The presence of macrophages and inflammatory responses in an in vitro testicular co-culture model of male reproductive development enhance relevance to in vivo conditions. Toxicology In Vitro 2016;36:210-215. |
R834514 (Final) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2016) R835738C004 (2017) |
Exit Exit |
|
|
Hofmann JN, Keifer MC, Furlong CE, De Roos AJ, Farin FM, Fenske RA, van Belle G, Checkoway H. Serum cholinesterase inhibition in relation to paraoxonase-1 (PON1) status among organophosphate-exposed agricultural pesticide handlers. Environmental Health Perspectives 2009;117(9):1402-1408. |
R834514 (2012) R834514 (2013) R831709 (Final) |
|
|
|
Hofmann JN, Keifer MC, De Roos AJ, Fenske RA, Furlong CE, van Belle G, Checkoway H. Occupational determinants of serum cholinesterase inhibition among organophosphate-exposed agricultural pesticide handlers in Washington State. Occupational and Environmental Medicine 2010;67(6):375-386. |
R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Hohl SD, Gonzalez C, Carosso E, Ibarra G, Thompson B. "I did it for us and I would do it again": perspectives of rural Latinos on providing biospecimens for research. American Journal of Public Health 2014;104(5):911-916. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Holme F, Thompson B, Holte S, Vigoren EM, Espinoza N, Ulrich A, Griffith W, Faustman EM. The role of diet in children's exposure to organophosphate pesticides. Environmental Research 2016;147:133-140. |
R834514 (Final) R834514C001 (2015) R834514C001 (Final) |
Exit Exit Exit |
|
|
Inoue S, Becker AL, Kim J-H, Shu Z, Soelberg SD, Weigel KM, Hiraiwa M, Cairns A, Lee H-B, Furlong CE, Oh K, Lee K-H, Gao D, Chung J-H, Cangelosi GA. Semi-automated, occupationally safe immunofluorescence microtip sensor for rapid detection of mycobacterium cells in sputum. PLoS One 2014;9(1):e86018. |
R834514 (Final) R834514C004 (2015) |
Exit Exit |
|
|
Jackson JE, Yost MG, Karr CJ, Fitzpatrick C, Lamb BK, Chung S, Chen J, Avise J, Rosenblatt RA, Fenske RA. Public health impacts of climate change in Washington State: projected mortality risks due to heat events and air pollution. Climatic Change 2010;102(1-2):159-186. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit |
|
|
Jansen KL, Cole TB, Park SS, Furlong CE, Costa LG. Paraoxonase 1 (PON1) modulates the toxicity of mixed organophosphorus compounds. Toxicology and Applied Pharmacology 2009;236(2):142-153. |
R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Karr CJ, Rudra CB, Miller KA, Gould TR, Larson T, Sathyanarayana S, Koenig JQ. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environmental Research 2009;109(3):321-327. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Karr CJ, Demers PA, Koehoorn MW, Lencar CC, Tamburic L, Brauer M. Influence of ambient air pollutant sources on clinical encounters for infant bronchiolitis. American Journal of Respiratory and Critical Care Medicine 2009;180(10):995-1001. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Karr CJ. Adding fuel to the fire: increasing evidence for developmental toxicity of indoor solid fuel combustion. Archives of Pediatric & Adolescent Medicine 2011;165(6):565-566. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Karr CJ. Children's environmental health in agricultural settings. Journal of Agromedicine 2012;17(2):127-139. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Karr C. Addressing environmental contaminants in pediatric practice. Pediatrics in Review 2011;32(5):190-200. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Richter RJ, Marshall JK, Eintracht JF, Rosenthal EA, Furlong CE, Jarvik GP. Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. Journal of Lipids 2012;2012:476316 (11 pp.). |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Richter RJ, Marshall JK, Nakayama KS, Jarvik ER, Eintracht JF, Rosenthal EA, Furlong CE, Jarvik JP. Dietary cholesterol increases paraoxonase 1 enzyme activity. Journal of Lipid Research 2012;53(11):2450-2458. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Kim DS, Burt AA, Crosslin DR, Robertson PD, Ranchalis JE, Boyko EJ, Nickerson DA, Furlong CE, Jarvik GP. Novel common and rare genetic determinants of paraoxonase activity: FTO, SERPINA12, and ITGAL. Journal of Lipid Research 2013;54(2):552-560. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Jarvik ER, Rosenthal EA, Hatsukami TS, Furlong CE, Jarvik GP. Novel gene-by-environment interactions: APOB and NPC1L1 variants affect the relationship between dietary and total plasma cholesterol. Journal of Lipid Research 2013;54(5):1512-1520. |
R834514 (2013) |
Exit Exit Exit |
|
|
Kim DS, Marsillach J, Furlong CE, Jarvik GP. Pharmacogenetics of paraoxonase activity: elucidating the role of high-density lipoprotein in disease. Pharmacogenomics 2013;14(12):1495-1515. |
R834514 (2013) R834514C004 (Final) |
Exit |
|
|
Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, Eintracht JF, Hatsukami TS, Furlong CE, Marcovina S, Albers JJ, Jarvik GP. HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. Journal of the American Heart Association 2014;3(3):e000902. |
R834514 (Final) R834514C004 (2015) |
Exit Exit |
|
|
Kim DS, Crosslin DR, Auer PL, Suzuki SM, Marsillach J, Burt AA, Gordon AS, Meschia JF, Nalls MA, Worrall BB, Longstreth Jr. WT, Gottesman RF, Furlong CE, Peters U, Rich SS, Nickerson DA, Jarvik GP. Rare coding variation in paraoxonase-1 is associated with ischemic stroke in the NHLBI Exome Sequencing Project. Journal of Lipid Research 2014;55(6):1173-1178. |
R834514 (Final) R834514C004 (2015) |
Exit Exit Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Jarvik LE, Eintracht JF, Furlong CE, Jarvik GP. Effects of dietary components on high-density lipoprotein measures in a cohort of 1,566 participants. Nutrition & Metabolism 2014;11(1):44 (9 pp.). |
R834514C004 (2015) |
Exit Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Vuletic S, Vaisar T, Li WF, Rosenthal EA, Dong W, Eintracht JF, Motulsky AG, Brunzell JD, Albers JJ, Furlong CE, Jarvik GP. PLTP activity inversely correlates with CAAD: effects of PON1 enzyme activity and genetic variants on PLTP activity. Journal of Lipid Research 2015;56(7):1351-1362. |
R834514 (Final) R834514C004 (2015) |
Exit Exit Exit |
|
|
Kim DS, Li YK, Bell GA, Burt AA, Vaisar T, Hutchins PM, Furlong CE, Otvos JD, Polak JF, Arnan MK, Kaufman JD, McClelland RL, Longstreth Jr WT, Jarvik GP. Concentration of smaller high-density lipoprotein particle (HDL-P) is inversely correlated with carotid intima media thickening after confounder adjustment: the Multi Ethnic Study of Atherosclerosis (MESA). Journal of the American Heart Association 2016;5(5):e002977. |
R834514 (Final) |
Exit Exit Exit |
|
|
Kim HY, Wegner SH, Van Ness KP, Park JJ, Pacheco SE, Workman T, Hong S, Griffith W, Faustman EM. Differential epigenetic effects of chlorpyrifos and arsenic in proliferating and differentiating human neural progenitor cells. Reproductive Toxicology 2016;65:212-223. |
R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Kim JH, Stevens RC, MacCoss MJ, Goodlett DR, Scherl A, Richter RJ, Suzuki SM, Furlong CE. Identification and characterization of biomarkers of organophosphorus exposures in humans. Advances in Experimental Medicine and Biology 2010;660:61-71. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit |
|
|
Kim JH, Yeo WH, Shu Z, Soelberg SD, Inoue S, Kalyanasundaram D, Ludwig J, Furlong CE, Riley JJ, Weigel KM, Cangelosi GA, Oh K, Lee KH, Gao D, Chung JH. Immunosensor towards low-cost, rapid diagnosis of tuberculosis. Lab on a Chip 2012;12(8):1437-1440. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Kim NJ, Vasquez VB, Torres E, Nicola RM, Karr C. Breaking the silence: sexual harassment of Mexican women farmworkers. Journal of Agromedicine 2016;21(2):154-162. |
R834514 (Final) |
Exit |
|
|
Kullman SW, Hamm JT, Hinton DE. Identification and characterization of a cDNA encoding cytochrome P450 3A from the fresh water teleost medaka (Oryzias latipes). Archives of Biochemistry and Biophysics 2000;380(1):29-38. |
R834514 (2011) R834514 (Final) R825298 (Final) |
Exit Exit |
|
|
Lanktree MB, Guo Y, Murtaza M, Glessner JT, Bailey SD, Onland-Moret NC, Lettre G, Ongen H, Rajagopalan R, Johnson T, Shen H, Nelson CP, Klopp N, Baumert J, Padmanabhan S, Pankratz N, Pankow JS, Shah S, Taylor K, Barnard J, Peters BJ, Maloney CM, Lobmeyer MT, Stanton A, Zafarmand MH, Romaine SP, Mehta A, van Iperen EP, Gong Y, Price TS, Smith EN, Kim CE, Li YR, Asselbergs FW, Atwood LD, Bailey KM, Bhatt D, Bauer F, Behr ER, Bhangale T, Boer JM, Boehm BO, Bradfield JP, Brown M, Braund PS, et al. Meta-analysis of dense genecentric association studies reveals common and uncommon variants associated with height. American Journal of Human Genetics 2011;88(1):6-18. |
R834514 (2011) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Liyasova M, Li B, Schopfer LM, Nachon F, Masson P, Furlong CE, Lockridge O. Exposure to tri-o-cresyl phosphate detected in jet airplane passengers. Toxicology and Applied Pharmacology 2011;256(3):337-347. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Loftus C, Yost M, Sampson P, Arias G, Torres E, Vasquez VB, Bhatti P, Karr C. Regional PM2.5 and asthma morbidity in an agricultural community: a panel study. Environmental Research 2015;136:505-512. |
R834514 (2015) R834514 (Final) R834514C002 (2015) R834514C002 (Final) |
Exit Exit Exit |
|
|
Loftus C, Yost M, Sampson P, Torres E, Arias G, Breckwich Vasquez V, Hartin K, Armstrong J, Tchong French M, Vedal S, Bhatti P, Karr C. Ambient ammonia exposures in an agricultural community and pediatric asthma morbidity. Epidemiology 2015;26(6):794-801. |
R834514 (2015) |
Exit |
|
|
MacIntyre EA, Karr CJ, Koehoorn M, Demers P, Tamburic L, Lencar C, Brauer M. Otitis media incidence and risk factors in a population-based birth cohort. Paediatrics and Child Health 2010;15(7):437-442. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
MacIntyre EA, Karr CJ, Koehoorn M, Demers PA, Tamburic L, Lencar C, Brauer M. Residential air pollution and otitis media during the first two years of life. Epidemiology 2011;22(1):81-89. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Mackenzie-Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicology and Teratology 2010;32(4):452-459. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Malen R, Knerr S, Delgado F, Fullerton SM, Thompson B. Rural Mexican-Americans’ perceptions of family health history, genetics, and disease risk: implications for disparities-focused research dissemination. Journal of Community Genetics 2016;7(1):91-96. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Marsillach J, Richter RJ, Kim JH, Stevens RC, MacCoss MJ, Tomazela D, Suzuki SM, Schopfer LM, Lockridge O, Furlong CE. Biomarkers of organophosphorus (OP) exposures in humans. NeuroToxicology 2011;32(5):656-660. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Marsillach J, Hsieh EJ, Richter RJ, MacCoss MJ, Furlong CE. Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chemico-Biological Interactions 2013;203(1):85-90. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Marsillach J, Costa LG, Furlong CE. Protein adducts as biomarkers of exposure to organophosphorus compounds. Toxicology 2013;307:46-54. |
R834514 (2013) R834514C004 (Final) |
Exit Exit |
|
|
Marsillach J, Suzuki SM, Richter RJ, McDonald MG, Rademacher PM, MacCoss MJ, Hsieh EJ, Rettie AE, Furlong CE. Human valacyclovir hydrolase/biphenyl hydrolase-like protein is a highly efficient homocysteine thiolactonase. PLoS One 2014;9(10):e110054. |
R834514 (Final) R834514C004 (2015) |
Exit Exit |
|
|
Marsillach J, Becker JO, Vaisar T, Hahn BH, Brunzell JD, Furlong CE, deBoer IH, McMahon MA, Hoofnagle AN, DCCT/EDIC Research Group. Paraoxonase-3 is depleted from the high-density lipoproteins of autoimmune disease patients with subclinical atherosclerosis. Journal of Proteome Research 2015;14(5):2046-2054. |
R834514 (Final) R834514C004 (2015) |
Exit Exit Exit |
|
|
Marsillach J, Costa LG, Furlong CE. Paraoxonase-1 and early-life environmental exposures. Annals of Global Health 2016;82(1):100-110. |
R834514 (Final) |
Exit Exit Exit |
|
|
McMillin MJ, Below JE, Shively KM, Beck AE, Gildersleeve HI, Pinner J, Gogola GR, Hecht JT, Grange DK, Harris DJ, Earl DL, Jagadeesh S, Mehta SG, Robertson SP, Swanson JM, Faustman EM, Mefford HC, Shendure J, Nickerson DA, Bamshad MJ, University of Washington Center for Mendelian Genomics. Mutations in ECEL1 cause distal arthrogryposis type 5D. American Journal of Human Genetics 2013;92(1):150-156. |
R834514 (2013) |
Exit Exit |
|
|
Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, Vokonas PS, Schwartz JD. Long-term exposure to ambient fine particulate matter and renal function in older men: the Veterans Administration Normative Aging Study. Environmental Health Perspectives 2016;124(9):1353-1360. |
R834514C004 (Final) R834798 (Final) R834798C002 (Final) |
|
|
|
Moreira EG, Yu X., Robinson JF, Griffith W, Hong SW, Beyer RP, Bammler TK, Faustman EM. Toxicogenomic profiling in maternal and fetal rodent brains following gestational exposure to chlorpyrifos. Toxicology and Applied Pharmacology 2010;245(3):310-325. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (2007) R831709 (Final) |
Exit Exit Exit |
|
|
Nonnenmann MW, Coronado G, Thompson B, Griffith WC, Hanson JD, Vesper S, Faustman EM. Utilizing pyrosequencing and quantitative PCR to characterize fungal populations among house dust samples. Journal of Environmental Monitoring 2012;14(8):2038-2043. |
R834514 (2013) R834514 (Final) |
Exit |
|
|
O’Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Reviews on Environmental Health 2008;23(3):167-202. |
R834514 (2011) R834514 (Final) EM833367 (2009) EM833367 (Final) |
Exit |
|
|
Paulson JA, Karr CJ, Seltzer JM, Cherry DC, Sheffield PE, Cifuentes E, Buka I, Amler RW. Development of the pediatric environmental health specialty unit network in North America. American Journal of Public Health 2009;99(Suppl 3):S511-S516. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Perkins A, Walters F, Sievert J, Rhodes B, Morrissey B, Karr CJ. Home use of a pyrethroid-containing pesticide and facial paresthesia in a toddler:a case report. International Journal of Environmental Research and Public Health 2016;13(8):829 (7 pp.). |
R834514 (Final) |
Exit Exit Exit |
|
|
Perla ME, Rue T, Cheadle A, Krieger J, Karr CJ. Population-based comparison of biomarker concentrations for chemicals of concern among Latino-American and non-Hispanic white children. Journal of Immigrant and Minority Health 2015;17(3):802-819. |
R834514 (2015) |
Exit |
|
|
Perla ME, Iman E, Campos L, Perkins A, Liebman AK, Miller ME, Beaudet NJ, Karr CJ. Agricultural occupational health and safety perspectives among Latino-American youth. Journal of Agromedicine 2015;20(2):167-177. |
R834514 (2015) |
|
|
|
Perla ME, Rue T, Cheadle A, Krieger J, Karr CJ. Biomarkers of insecticide exposure and asthma in children: a National Health and Nutrition Examination Survey (NHANES) 1999-2008 analysis. Archives of Environmental & Occupational Health 2015;70(6):309-322. |
R834514 (2015) |
Exit |
|
|
Pizzurro DM, Dao K, Costa LG. Astrocytes protect against diazinon- and diazoxon-induced inhibition of neurite outgrowth by regulating neuronal glutathione. Toxicology 2014;318:59-68. |
R834514C003 (Final) |
Exit Exit Exit |
|
|
Pizzurro DM, Dao K, Costa LG. Diazinon and diazoxon impair the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons. Toxicology and Applied Pharmacology 2014;274(3):372-382. |
R834514C003 (Final) |
Exit Exit Exit |
|
|
Plascak JJ, Molina Y, Wu-Georges S, Idris A, Thompson B. Latino residential segregation and self-rated health among Latinos: Washington State Behavioral Risk Factor Surveillance System, 2012-2014. Social Science & Medicine 2016;159:38-47. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Postma J, Karr C, Kieckhefer G. Community health workers and environmental interventions for children with asthma: a systematic review. Journal of Asthma 2009;46(6):564-576. |
R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Postma J, Younglove LR, Odom-Maryon T, Brooks K, Burbacher TM, Butterfield PW, Butterfield PG, Cederblom N, Grant K, Beresford SAA, Faustman EM. Hispanic representation in a longitudinal birth cohort study. Journal of Health Disparities Research and Practice 2016;9(2):66-77. |
R834514 (Final) |
Exit Exit |
|
|
Raanan R, Gunier RB, Balmes JR, Beltran AJ, Harley KG, Bradman A, Eskenazi B. Elemental sulfur use and associations with pediatric lung function and respiratory symptoms in an agricultural community (California, USA). Environmental Health Perspectives 2017;125(8):087007 (8 pp.). |
R834514 (Final) R826709 (2002) R831710 (Final) R834513 (Final) |
|
|
|
Ramaprasad J, Tsai MG, Fenske RA, Faustman EM, Griffith WC, Felsot AS, Elgethun K, Weppner S, Yost MG. Children's inhalation exposure to methamidophos from sprayed potato fields in Washington State:exploring the use of probabilistic modeling of meteorological data in exposure assessment. Journal of Exposure Science and Environmental Epidemiology 2009;19(6):613-623. |
R834514 (Final) R834514C002 (Final) R831709 (2007) R831709 (Final) |
Exit Exit |
|
|
Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicology and Applied Pharmacology 2009;235(1):1-9. |
R834514 (2012) R834514 (2013) R834514 (Final) R831709 (2007) R831709 (Final) |
Exit Exit Exit |
|
|
Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 status as a risk factor for disease or exposure. Advances in Experimental Medicine and Biology 2010;660:29-35. |
R834514 (2011) R834514 (2013) |
Exit |
|
|
Roberts JR, Karr CJ, Council on Environmental Health. Pesticide exposure in children. Pediatrics 2012;130(6):e1765-e1788. |
R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Roberts JR, Karr CJ, de Ybarrondo L, McCurdy LE, Freeland KD, Husley TC, Forman J. Improving pediatrician knowledge about environmental triggers of asthma. Clinical Pediatrics 2013;52(6):527-533. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Roberts JR, Newman N, McCurdy LE, Chang JS, Salas MA, Eskridge B, De Ybarrondo L, Sandel M, Mazur L, Karr CJ. Integrating environmental management of asthma into pediatric health care: what worked and what still needs improvement? Clinical Pediatrics 2016;55(14):1271-1278. |
R834514 (Final) |
Exit Exit |
|
|
Robinson JF, Guerrette Z, Yu X, Hong S, Faustman EM. A systems-based approach to investigate dose-and time-dependent methylmercury-induced gene expression response in C57BL/6 mouse embryos undergoing neurulation. Birth Defects Research. Part B: Developmental and Reproductive Toxicology 2010;89(3):188-200. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (Final) |
Exit |
|
|
Robinson JF, Yu X, Hong S, Zhou C, Kim N, DeMasi D, Faustman EM. Embryonic toxicokinetic and dynamic differences underlying strain sensitivity to cadmium during neurulation. Reproductive Toxicology 2010;29(3):279-285. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) R831709 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Port JA, Yu X, Faustman EM. Integrating genetic and toxicogenomic information for determining underlying susceptibility to developmental disorders. Birth Defects Research. Part A--Clinical and Molecular Teratology 2010;88(10):920-930. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit |
|
|
Robinson JF, Griffith WC, Yu X, Hong S, Kim E, Faustman EM. Methylmercury induced toxicogenomic response in C57 and SWV mouse embryos undergoing neural tube closure. Reproductive Toxicology 2010;30(2):284-291. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Yu X, Moreira EG, Hong S, Faustman EM. Arsenic-and cadmium-induced toxicogenomic response in mouse embryos undergoing neurulation. Toxicology and Applied Pharmacology 2011;250(2):117-129. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Theunissen PT, van Dartel DA, Pennings JL, Faustman EM, Piersma AH. Comparison of MeHg-induced toxicogenomic responses across in vivo and in vitro models used in developmental toxicology. Reproductive Toxicology 2011;32(2):180-188. |
R834514 (Final) R834514C003 (Final) |
Exit Exit Exit |
|
|
Sathyanarayana S, Basso O, Karr CJ, Lozano P, Alavanja M, Sandler DP, Hoppin JA. Maternal pesticide use and birth weight in the Agricultural Health Study. Journal of Agromedicine 2010;15(2):127-136. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Sathyanarayana S, Zhou C, Rudra CB, Gould T, Larson T, Koenig J, Karr CJ. Prenatal ambient air pollution exposure and small for gestational age birth in the Puget Sound Air Basin. Air Quality, Atmosphere & Health 2013;6(2):455-463. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Saxena R, Elbers CC, Guo Y, Peter I, Gaunt TR, Mega JL, Lanktree MB, Tare A, Castillo BA, Li YR, Johnson T, Bruinenberg M, Gilbert-Diamond D, Rajagopalan R, Voight BF, Balasubramanyam A, Barnard J, Bauer F, Baumert J, Bhangale T, Bohm BO, Braund PS, Burton PR, Chandrupatla HR, Clarke R, Cooper-DeHoff RM, Crook ED, Davey-Smith G, Day IN, de Boer A, de Groot MC, Drenos F, Ferguson J, Fox CS, Furlong CE, Gibson Q, Gieger C, Gilhuijs-Pederson LA, Glessner JT, Goel A, Gong Y, Grant SF, Grobbee DE, Hastie C, Humphries SE, Kim CE, Kivimaki M, Kleber M, Meisinger C, Kumari M, Langaee TY, Lawlor DA, Li M, Lobmeyer MT, Maitland-van der Zee AH, Meijs MF, Molony CM, Morrow DA, Murugesan G, Musani SK, Nelson CP, Newhouse SJ, O'Connell JR, Padmanabhan S, Palmen J, Patel SR, Pepine CJ, Pettinger M, Price TS, Rafelt S, Ranchalis J, Rasheed A, Rosenthal E, Ruczinski I, Shah S, Shen H, Silbernagel G, Smith EN, Spijkerman AW, Stanton A, Steffes MW, Thorand B, Trip M, van der Harst P, van der A DL, van Iperen EP, van Setten J, van Vliet-Ostaptchouk JV, Verweij N, Wolffenbuttel BH, Young T, Zafarmand MH, Zmuda JM; Look AHEAD Research Group; DIAGRAM consortium, Boehnke M, Altshuler D, McCarthy M, Kao WH, Pankow JS, Cappola TP, Sever P, Poulter N, Caulfield M, Dominiczak A, Shields DC, Bhatt DL, Zhang L, Curtis SP, Danesh J, Casas JP, van der Schouw YT, Onland-Moret NC, Doevendans PA, Dorn GW 2nd, Farrall M, FitzGerald GA, Hamsten A, Hegele R, Hingorani AD, Hofker MH, Huggins GS, Illig T, Jarvik GP, Johnson JA, Klungel OH, Knowler WC, Koenig W, März W, Meigs JB, Melander O, Munroe PB, Mitchell BD, Bielinski SJ, Rader DJ, Reilly MP, Rich SS, Rotter JI, Saleheen D, Samani NJ, Schadt EE, Shuldiner AR, Silverstein R, Kottke-Marchant K, Talmud PJ, Watkins H, Asselbergs FW, de Bakker PI, McCaffery J, Wijmenga C, Sabatine MS, Wilson JG, Reiner A, Bowden DW, Hakonarson H, Siscovick DS, Keating BJ. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. American Journal of Human Genetics 2012;90(3):410-425. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Schopfer LM, Furlong CE, Lockridge O. Development of diagnostics in the search of an explanation of aerotoxic syndrome. Analytical Biochemistry 2010;404(1):64-74. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Serrano SE, Karr CJ, Seixas NS, Nguyen RH, Barrett ES, Janssen S, Redmon B, Swan SH, Sathyanarayana S. Dietary phthalate exposure in pregnant women and the impact of consumer practices. International Journal of Environmental Research and Public Health 2014;11(6):6193-6215. PMID:24927036. |
R834514 (2015) |
Exit Exit |
|
|
Shepherd-Banigan M, Ulrich A, Thompson B. Macro-activity patterns of farmworker and non-farmworker children living in an agricultural community. Environmental Research 2014;132:176-181. |
R834514 (Final) R834514C001 (Final) |
Exit Exit Exit |
|
|
Shepherd-Banigan M, Hohl SD, Vaughan C, Ibarra G, Carosso E, Thompson B. "The promotora explained everything": participant experiences during a household-level diabetes education program. The Diabetes Educator 2014;40(4):507-515. |
R834514C001 (2015) |
Exit |
|
|
Smith MN, Griffith WC, Beresford SA, Vredevoogd M, Vigoren EM, Faustman EM. Using a biokinetic model to quantify and optimize cortisol measurements for acute and chronic environmental stress exposure during pregnancy. Journal of Exposure Science and Environmental Epidemiology 2014;24(5):510-516. |
R834514 (2013) R834514 (Final) |
Exit |
|
|
Smith MN, Wilder CS, Griffith WC, Workman T, Thompson B, Dills R, Onstad G, Vredevoogd M, Vigoren EM, Faustman EM. Seasonal variation in cortisol biomarkers in Hispanic mothers living in an agricultural region. Biomarkers 2015;20(5):299-305. |
R834514 (2015) R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Smith MN, Grice J, Cullen A, Faustman EM. A toxicological framework for the prioritization of Children’s Safe Product Act data. International Journal of Environmental Research and Public Health 2016;13(4):431 (24 pp.). |
R834514 (Final) R835738 (2016) R835738 (2017) R835738C005 (2015) R835738C005 (2016) R835738C005 (2017) |
Exit Exit Exit |
|
|
Smith MN, Workman T, McDonald KM, Vredevoogd MA, Vigoren EM, Griffith WC, Thompson B, Coronado GD, Barr D, Faustman EM. Seasonal and occupational trends of five organophosphate pesticides in house dust. Journal of Exposure Science and Environmental Epidemiology 2017;27(4):372-378. |
R834514 (Final) |
Exit |
|
|
Smith M, Hubal E, Faustman E. A Case study on the utility of predictive toxicology tools in alternatives assessments for hazardous chemicals in children's consumer products. JOURNAL OF EXPOSURE SCIENCE AND ENVIRONMENTAL EPIDEMIOLOGY 2020;30(1):160-170. |
R834514 (Final) |
Exit |
|
|
Souza-Nogueira A, Camargo AE, Remondi FA, Paoliello MM, Richter RJ, Furlong CE, Barbosa DS, Maes M, Moreira EG. Paraoxonase 1 (PON1) Q192R genotypes and their interaction with smoking strongly increase atherogenicity and the Framingham risk score. Archives of Endocrinology and Metabolism 2016;60(5):426-435. |
R834514 (Final) |
Exit Exit |
|
|
Sridharan G, Wamalwa D, John-Stewart G, Tapia K, Langat A, Moraa Okinyi H, Adhiambo J, Chebet D, Maleche-Obimbo E, Karr CJ, Benki-Nugent S. High viremia and wasting before antiretroviral therapy are associated with pneumonia in early-treated HIV-infected Kenyan infants. Journal of the Pediatric Infectious Diseases Society 2017;6(3):245-252. |
R834514 (Final) |
Exit Exit |
|
|
Stanaway IB, Wallace JC, Shojaie A, Griffith WC, Hong S, Wilder CS, Green FH, Tsai J, Knight M, Workman T, Vigoren EM, McLean JS, Thompson B, Faustman EM. Human oral buccal microbiomes are associated with farmworker status and azinphos-methyl agricultural pesticide exposure. Applied and Environmental Microbiology 2017;83(2):e02149-16. |
R834514 (Final) |
Exit Exit Exit |
|
|
Stanaway I, Wallace J, Hong S, WIlder C, Green F, Tsai J, Knight M, Workman T, Vigoren E, Smith M, Griffith W, Thompson B, Shojaie A, Faustman E. Alteration of oral microbiome composition in children living with pesticide-exposed farm workers. INTERNATIONAL JOURNAL OF HYGIENE AND ENVIRONMENTAL HEALTH 2023;248(114090). |
R834514 (Final) R832733 (Final) |
Exit |
|
|
Strong LL, Thompson B, Koepsell TD, Meischke H, Coronado GD. Reducing the take-home pathway of pesticide exposure:behavioral outcomes from the Para Niños Saludables study. Journal of Occupational and Environmental Medicine 2009;51(8):922-933. |
R834514 (Final) R834514C001 (Final) R831709 (2007) R831709 (Final) |
Exit |
|
|
Strong LL, Starks HE, Meischke H, Thompson B. Perspectives of mothers in farmworker households on reducing the take-home pathway of pesticide exposure. Health Education & Behavior 2009;36(5):915-929. |
R834514 (Final) R834514C001 (Final) |
Exit Exit Exit |
|
|
Suzuki SM, Stevens RC, Richter RJ, Cole TB, Park S, Otto TC, Cerasoli DM, Lenz DE, Furlong CE. Engineering human PON1 in an E. coli expression system. Advances in Experimental Medicine and Biology 2010;660:37-45. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) R831709 (Final) |
Exit |
|
|
Thompson B, Griffith WC, Barr DB, Coronado GD, Vigoren EM, Faustman EM. Variability in the take-home pathway: farmworkers and non-farmworkers and their children. Journal of Exposure Science & Environmental Epidemiology 2014;24(5):522-531. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Ticozzi N, LeClerc AL, Keagle PJ, Glass JD, Wills AM, van Blitterswijk M, Bosco DA, Rodriguez-Levva I, Gellera C, Ratti A, Taroni F, McKenna-Yasek D, Sapp PC, Silani V, Furlong CE, Brown Jr. RH, Landers JE. Paraoxonase gene mutations in amyotrophic lateral sclerosis. Annals of Neurology 2010;68(1):102-107. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit |
|
|
Wegner SH, Yu X, Pacheco Shubin S, Griffith WC, Faustman EM. Stage-specific signaling pathways during murine testis development and spermatogenesis: a pathway-based analysis to quantify developmental dynamics. Reproductive Toxicology 2015;51:31-39. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2017) |
Exit Exit Exit |
|
|
Wegner S, Hong S, Yu X, Faustman EM. Preparation of rodent testis co-cultures. Current Protocols in Toxicology 2013;Chapter 16:Unit 16.10. |
R834514 (2013) |
Exit |
|
|
Wegner S, Yu X, Kim HY, Harris S, Griffith WC, Hong S, Faustman EM. Effect of dipentyl phthalate in 3-dimensional in vitro testis co-culture is attenuated by cyclooxygenase-2 inhibition. Journal of Toxicology and Environmental Health Sciences 2014;6(8):161-169. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit |
|
|
Wegner S, Park J, Workman T, Workman T, Hermsan S, Wallace J, Stanaway I, Kim H, Griffith W, Hong S, Faustman E. Anchoring a dynamic in vitro model of human neuronal differentiation to key processes of early brain development in vivo. Reproductive Toxicology 2020;91:116-130. |
R834514 (Final) R835738 (2018) |
Exit Exit |
|
|
Wegner S, Workman T, Park J, Harris S, Wallace J, Stanaway I, Hong S, Hansen B, Griffith W, Faustman E. A dynamic in vitro developing testis model reflects structures and functions of testicular development in vivo. REPRODUCTIVE TOXICOLOGY 2023;118(108362) |
R834514 (Final) R835738 (Final) |
Exit |
|
|
Weiner MW, Meyerhoff DJ, Neylan TC, Hlavin J, Ramage ER, McCoy D, Studholme C, Cardenas V, Marmar C, Truran D, Chu PW, Kornak J, Furlong CE, McCarthy C. The relationship between Gulf War war illness, brain N-acetylaspartate, and post-traumatic stress disorder. Military Medicine 2011;176(8):896-902. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit |
|
|
Weldon BA, Shubin SP, Smith MN, Workman T, Artemenko A, Griffith WC, Thompson B, Faustman EM. Urinary microRNAs as potential biomarkers of pesticide exposure. Toxicology and Applied Pharmacology 2016;312:19-25. |
R834514 (Final) R834514C001 (Final) |
Exit Exit Exit |
|
|
Whitely Binder LC, Barcelos JK, Booth DB, Darzen M, Elsner MM, Fenske R, Graham TF, Hamlet AF, Hodges-Howell J, Jackson JE, Karr C, Keys PW, Littell JS, Mantua N, Marlow J, McKenzie D, Robinson-Dorn M, Rosenberg EA, Stockle CO, Vano JA. Preparing for climate change in Washington State. Climatic Change 2010;102(1-2):351-376. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit |
|
|
Woodruff TJ, Parker JD, Darrow LA, Slama R, Bell ML, Choi H, Glinianaia S, Hoggatt KJ, Karr CJ, Lobdell DT, Wilhelm M. Methodological issues in studies of air pollution and reproductive health. Environmental Research 2009;109(3):311-320. |
R834514 (2012) R834514 (2013) R834514 (Final) R833863 (2009) |
Exit Exit Exit |
|
|
Yu X, Robinson JF, Sidhu JS, Hong S, Faustman EM. A system-based comparison of gene expression reveals alterations in oxidative stress, disruption of ubiquitin-proteasome system and altered cell cycle regulation after exposure to cadmium and ethylmercury in mouse embryonic fibroblast. Toxicological Sciences 2010;114(2):356-377. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (2007) R831709 (Final) |
Exit Exit |
|
|
Yu X, Sidhu JS, Hong S, Robinson JF, Ponce RA, Faustman EM. Cadmium induced p53-dependent activation of stress signaling, accumulation of ubiquitinated proteins, and apoptosis in mouse embryonic fibroblast cells. Toxicological Sciences 2011;120(2):403-412. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R831709 (Final) |
Exit Exit |
|
|
Zachek CM, Karr CJ, Daniell W, Sweeney C, Miller MD. A network of Pediatric Environmental Health Specialty Units (PEHSUs): filling a critical gap in the health care system. Medycyna Środowiskowa--Environmental Medicine 2012;15(3):9-20. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Zhou C, Chen J, Zhang X, Costa LG, Guizzetti M. Prenatal ethanol exposure up-regulates the cholesterol transporters ATP-binding cassette A1 and G1 and reduces cholesterol levels in the developing rat brain. Alcohol and Alcoholism 2014;49(6):626-634. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Marsillach, J., E.J. Hsieh, R.J. Richter, M.J. Maccoss, and C.E. Furlong, Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chem Biol Interact, 2012. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Crosslin, DR, Auer, PL, Suzuki, SM, Marsillach, J, Burt, AA, Gordon, AS, Meschia, JF, Nalls, MA, Worrall, BB, Longstreth, WT, Jr., Gottesman, RF, Furlong, CE, Peters, U, Rich, SS, Nickerson, DA and Jarvik, GP. 2014. Rare coding variation in paraoxonase-1 is associated with ischemic stroke in the NHLBI Exome Sequencing Project. J Lipid Res. 55(6):1173-1178. 4031948. |
R834514C004 (Final) |
not available |
|
|
Guizzetti, M., N.H. Moore, G. Giordano, K.L. VanDeMark, and L.G. Costa, Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia, 2010. 58(12):p. 1395-406. PMCID2925144. |
R834514C004 (Final) |
not available |
|
|
Richter RJ, Jarvik GP, Furlong CE. 2010. Paraoxonase 1 (PON1) Status as a Risk Factor for Disease or Exposure. Adv Exp Med Biol 660:29-35. PMID 20221868. DOI 10.1007/978-1-60761-350-3_. NIHMSID #193805. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Burt, A, Ranchalis, J, Richter, RJ, Marshall, E, Rosenthal, E, Furlong, C and Jarvik, GP. 2012. Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. J Lipids 2012:476316. PMCID:PMC3364586. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Burt, AA, Ranchalis, JE, Richter, RJ, Marshall, JK, Nakayama, KS, Jarvik, ER, Eintracht, JF, Rosenthal, EA, Furlong, CE and Jarvik, GP. 2012. Dietary cholesterol increases paraoxonase 1 enzyme activity. J Lipid Res. 53(11):2450-8. PMCID:PMC3466014. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Burt, AA, Crosslin, DR, Robertson, PD, Ranchalis, JE, Boyko, EJ, Nickerson, DA, Furlong, CE and Jarvik, GP. 2013. Novel common and rare genetic determinants of paraoxonase activity:FTO, SERPINA12, and ITGAL. J Lipid Res. 54(2):552-60. PMCID:PMC3588879. |
R834514C004 (Final) |
not available |
|
|
Costa, LG, de Laat, R, Dao, K, Pellacani, C, Cole, TB and Furlong, CE. 2013. Paraoxonase-2 (PON2) in brain and its potential role in neuroprotection. Neurotoxicology. 3942372. |
R834514C004 (Final) |
not available |
|
|
Marsillach, J, Hsieh, EJ, Richter, RJ, MacCoss, MJ, Furlong, CE. 2013. Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chem Biol Interact 203(1):85-90. |
R834514C004 (Final) |
not available |
|
|
Costa, LG, Tait, L, de Laat, R, Dao, K, Giordano, G, Pellacani, C, Cole, TB and Furlong, CE. 2013. Modulation of paraoxonase 2 (PON2) in mouse brain by the polyphenol quercetin:a mechanism of neuroprotection? Neurochem Res. 38(9):1809-18. 3735620. |
R834514C004 (Final) |
not available |
|
|
Costa, LG, Giordano, G, Cole, TB, Marsillach, J and Furlong, CE. 2013. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology. 307(10):115-122. PMCID:PMC3516631. |
R834514C004 (Final) |
not available |
|
|
Cole, TB, Li, WF, Co, AL, Hay, AM, MacDonald, JW, Bammler, TK, Farin, FM, Costa, LG and Furlong, CE. 2014. Repeated Gestational Exposure of Mice to Chlorpyrifos Oxon is Associated with Paraoxonase 1 (PON1)-Modulated Effects in Maternal and Fetal Tissues. Toxicol Sci. |
R834514C004 (Final) |
not available |
|
|
Furlong CE, Marsillach J, Jarvik GP, Costa LG. 2016. Paraoxonases-1,-2 and-3:What are their functions? Chem Biol Interact. doi:10.1016/j.cbi.2016.05.036. PMID:27238723. |
R834514C004 (Final) R834513C003 (2016) |
not available |
|
|
Garrick JM, Dao K, de Laat R, Elsworth J, Cole TB, Marsillach J, Furlong CE, Costa LG. 2016. Developmental expression of paraoxonase 2. Chem Biol Interact. pii:S0009-2797(16)30116-8. doi:10.1016/j.cbi.2016.04.001. PMID:27062895. |
R834514C004 (Final) |
not available |
|
|
Marsillach J, Costa LG, Furlong CE. 2016. Paraoxonase-1 and Early-Life Environmental Exposures. Ann Glob Health. 82(1):100-10. PMID:27325068; PMCID:PMC4916371. |
R834514C004 (Final) |
not available |
|
|
Cole, T.B., J.C. Fisher, T.M. Burbacher, L.G. Costa, and C.E. Furlong, Neurobehavioral assessment of mice following repeated postnatal exposure to chlorpyrifos-oxon. Neurotoxicol Teratol, 2012. 34(3):p. 311-322. |
R834514C004 (Final) R834798C003 (Final) |
not available |
|
|
Burton C, Marsillach J, Zhang YS, Zhao W, Zheng X. Bioanalysis. 2015;7(13):1667-73. doi:10.4155/bio.15.90. Epub 2015 Jul 7** Judit Marsillach selected as a finalist for the Young Investigator Award to identify and reward promising early career researchers in the community. |
R834514C004 (Final) |
not available |
|
|
Furlong, C.E., R.J. Richter, L.G. Costa, and G.P. Jarvik, Paraoxonase 1 (PON1) Status in Risk Assessment for Organophosphate Exposure and Pharmacokinetics, in Chapter 9:Parameters for Pesticide QSAR and PBPK/PD Models for Human Risk Assessment. 2012, American Chemical Society:Available at:http://pubs.acs.org/doi/abs/10.1021/bk-2012-1099.ch009. p. 133-147. |
R834514C004 (Final) |
not available |
|
|
Costa, L.G., G. Giordano, and C.E. Furlong, Pharmacological and dietary modulators of paraxonase 1 (PON1) activity and expression:the hunt goes on. Biochem Pharmacol, 2011. 81(3):p. 337-344. PMCID3077125. |
R834514C004 (Final) |
not available |
|
|
Kim, D.S., A.A. Burt, J.E. Ranchalis, R.J. Richter, J.K. Marshall, K.S. Nakayama, E.R. Jarvik, J.F. Eintracht, E.A. Rosenthal, C.E. Furlong, and G.P. Jarvik, Dietary cholesterol increases paraoxonase 1 enzyme activity. J Lipid Res, 2012. 53(11):p. 2450-8. |
R834514C004 (Final) |
not available |
|
|
Kim, D.S., A. Burt, J. Ranchalis, R.J. Richter, E. Marshall, E. Rosenthal, C. Furlong, and G.P. Jarvik, Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. J Lipids, 2012. 2012(http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3364586/pdf/JL2012-476316.pdf). |
R834514C004 (Final) |
not available |
Supplemental Keywords:
Children’s health, environmental health, pesticide, environmental exposure, risk assessment, risk management, risk communication, community based participatory research, CBPR, intervention, Washington State, outreach and translation, mechanisms, susceptibility, genetics, gene-environment interactions, modeling, biostatistics, exposure assessment, exposure pathway, Health, RFA, Scientific Discipline, INTERNATIONAL COOPERATION, ENVIRONMENTAL MANAGEMENT, Environmental Policy, Biology, Children's Health, Biochemistry, Risk Assessment, biological markers, pesticides, age-related differences, pesticide exposure, agricultural community, children's vulnerablityProgress and Final Reports:
Original Abstract Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R834514C001 Community-Based Participatory Research
R834514C002 Pesticide Exposure Pathways
R834514C003 Molecular Mechanisms
R834514C004 Genetic Susceptibility
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2015 Progress Report
- 2014
- 2012 Progress Report
- 2011 Progress Report
- 2010
- Original Abstract
178 journal articles for this center