Grantee Research Project Results
Final Report: Center for Child Environmental Health Risks Research
EPA Grant Number: R834514Center: Predictive Toxicology Center for Organotypic Cultures and Assessment of AOPs for Engineered Nanomaterials
Center Director: Faustman, Elaine
Title: Center for Child Environmental Health Risks Research
Investigators: Faustman, Elaine , Fenske, Richard , Griffith, William C. , Yost, Michael , Costa, Lucio G , Furlong, Clement , Thompson, Engelberta , Vigoren, Eric M. , Karr, Catharine J.
Institution: University of Washington
EPA Project Officer: Callan, Richard
Project Period: September 25, 2009 through September 24, 2016
Project Amount: $5,417,075
RFA: Children's Environmental Health and Disease Prevention Research Centers (with NIEHS) (2009) RFA Text | Recipients Lists
Research Category: Children's Health , Human Health
Objective:
Since 1998, researchers of the University of Washington Center for Child Environmental Health Risks Research (the Center) have been using a multidisciplinary research approach working in the lab, in the field, and in the community to understand the mechanisms that define children’s susceptibility to pesticides, identify the implications of this susceptibility for development and learning, and partner with our communities to translate our findings into risk communication, risk management, and prevention strategies.
The Center has received a third cycle of funding from the National Institute of Environmental Health Sciences (NIEHS) and Environmental Protection Agency (EPA): approximately 30% and 70% of the total Center funds from the NIEHS and EPA, respectively.
The Center is administratively housed within the Institute for Risk Analysis and Risk Communication, also directed by Center Director Dr. Elaine M. Faustman, which is in the University of Washington’s School of Public Health. The Center includes partnerships with the Fred Hutchinson Cancer Research Center and the Yakima Valley community, located in the agricultural center of Washington State, to jointly sponsor a Community Based Participatory Research (CBPR) Project aimed at reducing childhood pesticide exposure.
All Center efforts were highly integrated and comprised two field-based research projects, two laboratory-based research projects, three facility cores, an Administrative Core, and Faculty Development Investigator.
The specific objectives of the two field-based projects—the CBPR project and the pesticide exposure pathways research project—were:
- To improve our understanding of critical pathways of potential pesticide exposure for children; and
- To apply culturally-appropriate interventions to reduce children’s exposure to pesticides.
The specific objectives of the laboratory-based research projects—a molecular mechanism research project and a genetic susceptibility research project—were:
- To identify cellular, biochemical, and molecular mechanisms that cause adverse developmental neurotoxicity of pesticides; and
- To identify susceptibility factors for developmental neurotoxicity of pesticides.
The three facility cores were: Biomarkers and Exposure Assessment (BEA), Biostatistics, Modeling and Risk Characterization (BMRC), and Community Outreach and Translation (COTC). The cores were designed to support the research objectives and to put the research into a child-specific risk assessment context. Thus, the specific objectives of the facility cores were:
- To provide core support for the development and application of risk assessment methods, enabling basic research on pesticide toxicity and exposure to inform risk decisions to protect children’s health from pesticides; and
- To foster partnerships between academic researchers and the community in which information requested by the community and basic research deficiencies/gaps are translated into studies that address the needs of both.
The Administrative Core provided fiscal oversight, resource management, coordination, and integration of Center activities. The COTC shared membership with the Administrative Core and included Dr. Catherine Karr, who since 2009 served as the Center’s Faculty Development Investigator, as well as Pediatric Health Specialist. Center support and mentoring enabled Dr. Karr to capitalize on the complementary and collaborative resources at the Center in order to navigate the transition to independent investigator in this subject area.
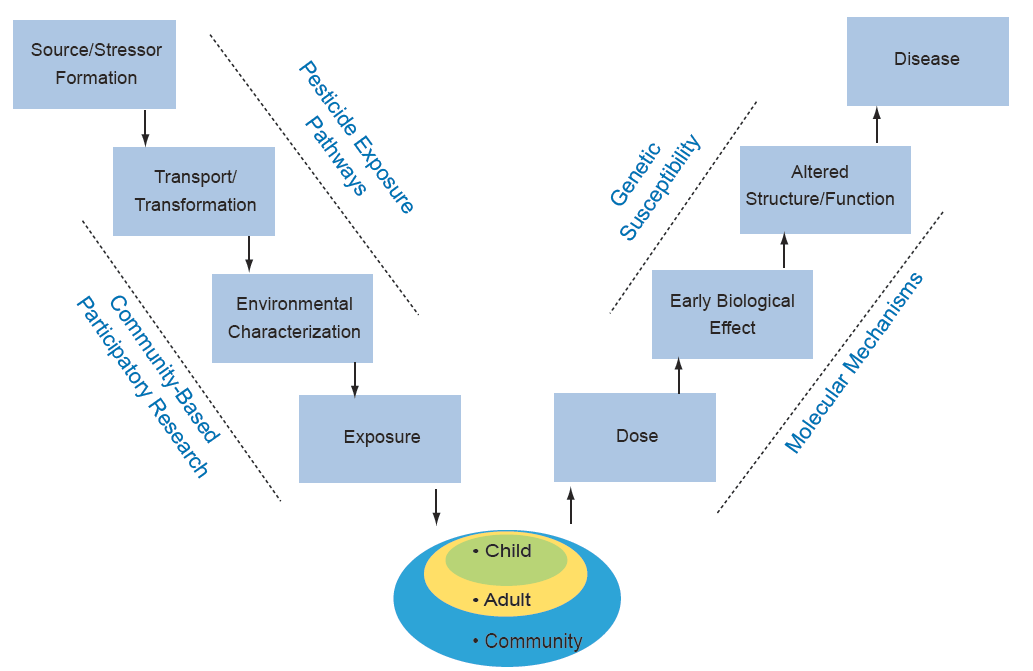
Work in the Center is organized around the Public Health paradigm “V-diagram,” which connects occurrence of disease in humans to the original source of the problem (see Figure 1). Along the pathway from source/stressor to disease, the diagram identifies intermediate processes (which may be subject to public health intervention) and conditions (which may be observable for public health monitoring and hypothesis testing). This paradigm is central to the overall organization and integration of the various components of the Center. Using Global Positioning System (GPS) tools to relate human activities and land use helps identify how organophosphate (OP) pesticide (stressor) application is transferred/transported to create OP environmental conditions. The CBPR Project focuses on the multiple potential pathways of OP transfer potentially leading to exposure and resulting dose in children. The biological sampling efforts help identify susceptibility factors and genetic mechanisms relating dose to early biological effect. Biostatistical analyses and outreach link all these components together.
Summary/Accomplishments (Outputs/Outcomes):
Community Based Participatory Research Project
In February 2011, our community health promoters began re-contacting farmworkers and non-farm workers in our cohort (from Years 05 through 09 studies) to participate in the new study. A total of 132 farmworkers and 90 non-farm workers were re-contacted before we were able to reach our recruitment goal of 60 farmworker and 40 non-farmworker households. Data collection began on March 17, 2011.
Data collection for Season 1 (Pre-Thinning season) was completed in late May 2011, Season 2 (Thinning season) data collection took place June - August 2011, and the final season of data collection (Non-spray season) occurred in November and December of 2011. During Season 2, we asked female participants, between 18 and 49 years of age, who were participating in the parent study, if they would be interested in participating in an additional Stress and Cortisol Project. We successfully recruited 27 women. We also coordinated with the Pesticides Exposure Pathways Research group to complete indoor and outdoor air sampling.
In the spring of 2012, a random selection of 41 participants from the larger CBPR Project were re-contacted to participate in one-on-one interviews to learn more about why they chose to provide biological samples for research and their experience in doing so. We learned that Hispanics have a strong understanding of and desire to contribute to biomedical research. Most participants were eager to participate because of self-described duty to help others and used words like “proud,” “satisfied,” and “pleasure” when describing their ability to contribute to a cause like this.
In February 2013, we held two Community Forums (one in English with the Community Advisory Board members and one in Spanish open to the community). During the forums, we provided examples of information from this project that would be available for dissemination at a later date. We then queried the audience on the presentation and understandability of the information. The information gathered during these meetings informed the dissemination tools that were used to return individual results to participants in June - July 2015. During this time, all 100 families (60 farm worker and 40 non-farm worker families) were re-contacted and offered a one-on-one meeting with one of our community health promoters to learn about the DMTP levels detected in their urine during the study, as well as more information on pesticides and reducing pesticide exposure at home and in the work place. We were successful in locating and returning results to 92 of the 100 participating adults; eight were lost to follow-up (moved out of the area). The 92 participants who received their results also completed a short survey that will assist us in better understanding the nature of their work environment and potential points of pesticide exposure. The majority of participants who met with a community health worker to discuss their results voiced great appreciation for the results and information provided to them.
Finally, in March of 2016, we held a Community Advisory Board meeting and a dissemination meeting where we invited participants to come and learn about the latest information in Washington State on pesticide exposure. Tito Rodriguez, a CAB member and a member of the Department of Health, spoke about acute exposures and what was being done to track and investigate such exposures. Ofelio Borges of the Department of Agriculture, another CAB member, gave a presentation on the new procedures for training farmworkers to be aware of pesticide exposure and to protect themselves and their families from exposure. We asked the participants of the study to complete a short survey that asked them what they remembered about the feedback they received as well as the protective practices we left with them. We received a total of 35 questionnaires. These data currently are being analyzed.
Nine months after distributing individual study results of urinary pesticide metabolites, we re-contacted 37 participants (who agreed to future contact) to assess the effectiveness of the dissemination process. An interview survey was used to assess if participants recalled a visit from a health promoter to discuss individual results and assessed their ability to correctly read the graphic created to display results. The survey also measured participants’ knowledge on pesticide protective practices, which was discussed during the dissemination of results. Almost all participants (97.3%) recalled a home visit from a health promoter to discuss their pesticide levels; twenty-nine (78.4%) correctly recalled that the health promoter used a thermometer or graphic to explain the results; twenty-six (70.3%) correctly interpreted graphics showing high and low exposure levels in adults and 75.7% correctly interpreted results for children. Multiple materials also were distributed with study results and the majority of participants recalled receiving the materials (81% - 92%), reviewed the materials (70% - 86%%), referred to the materials with questions regarding pesticides (54% - 73%), and shared the materials with family, friends or doctors (62% - 65%). Our study results support the use of a community based participatory research (CBPR) approach to decide how to best depict and disseminate study results. We found that this design was effective in explaining complicated study results to participants who often are left out of the dissemination process.
Significance. Recruitment and retention efforts were extremely successful in both the parent study and the Stress and Cortisol Project. Of the 100 families in the parent study, we lost only one non-farm worker family in Season 2, due to military service; and two farm worker families in Season 3 because they relocated out of state. Participants were very interested and enthusiastic about receiving individual results and appreciated the one-on-one meeting with the health worker who explained the results and dissemination materials in great deal. They also participated in the dissemination meeting where they learned more about what is being done in the state of Washington to protect workers and their families.
Pesticide Exposure Pathways Research Project
Summary statistics indicate that among the 181 households in the CHC 3 cohort, 125, 125, and 79 were located within 2000 meters of cherry, apple, and pear orchards, respectively. Within the same distance, arithmetic mean area (km2) for any crop type was largest for field corn [Mean 1.52 (SD: 1.15)], hops [0.55 (0.87)], and alfalfa hay [0.50 (0.40)]. Among tree fruit, mean area was largest for cherries [0.39 (0.58)], apples [0.32 (0.51)], and pears [0.31 (0.48)]. Preliminary univariate model results confirm our previous finding (Armstrong, 2014) that residences proximal to fields experience higher monthly outdoor levels of chlorpyrifos than non-proximal households. The 2000 and 4000 meter buffer sizes were found to be most predictive of the relationship between location and outdoor CPF concentration. On average, each 10-meter increase in residential distance from the nearest tree fruit orchard resulted in a 0.09% reduction in geometric mean outdoor CPF air concentration (ng/m3) (95% CI: 0.05, 0.14) (R2 = 0.48). Residential outdoor CPF air concentration was weakly correlated with mass of CPF applied within 250 meters (1.34, 95% CI: 1.10, 1.65, R2 = 0.12), but more strongly correlated with mass applied within 2000 meters (1.008, 95% CI: 1.004, 1.012, R2 = 0.55).
Significance. Our PLS LUR model adequately captured (R2=0.61) the variability in the 2011 outdoor air concentrations of CPF based on proximity to parcel level crop maps. Component 1 loaded heavily for distance from nearest tree fruit orchard and mass of chlorpyrifos applied within the largest buffers, component 2 loaded heavily for elevation and distance from nearest crop of any type, and component 3 loaded heavily for area of pears and corn within largest buffers. Geographic covariates that were most important for explaining chlorpyrifos variability were related to larger buffers (1-4 km).
The discrepancy of larger buffers being more predictive might be attributable to the fact that fewer houses (n=8) had any chlorpyrifos applied within the smaller buffer compared to the larger buffer (n=23), but it might also indicate that the off-target movement of airborne chlorpyrifos occurs over greater distances than previously thought. Farmworker status does not appear to be predictive of measured outdoor CPF levels in the full model. Previous significant findings related to farmworker status were likely due to farmworkers living closer to applied fields on average in the sample of homes.
Molecular Mechanisms Research Project
Aims of this research project are carried out in a collaborative manner by two laboratories, the Faustman Lab and the Costa Lab. Studies and results are reported herein for these joint efforts.
In the years preceding this latest funding cycle, Dr. Costa’s laboratory had been exploring novel mechanisms by which OP insecticides may adversely influence brain development. Two important findings were that OPs were able to inhibit proliferation of astrocytes, particularly in the presence of mitogens such as carbachol (an analog of acetylcholine) (Giordano, et al., 2005), and that the cytotoxicity of OPs on neurons was due to their ability to induce oxidative stress (Giordano, et al., 2007). For this latter study, we utilized an in vitro system consisting of cerebellar granule neurons (CGNs) isolated from wild-type mice (Gclm +/+) or from mice lacking the modifier subunit of glutamate cysteine ligase (Gclm -/-), the first and limiting step in the synthesis of glutathione. These neurons display very low levels of glutathione and are more susceptible to the toxicity of agents that increase oxidative stress.
During the same period, the Costa lab studied the interactions of astrocytes and neurons in mediating chemical toxicity. Various in vitro systems were implemented to study such interactions and a preliminary characterization of a number of parameters was carried out. Rat cortical or hippocampal astrocytes, when co-cultured with hippocampal neurons, increase their differentiation, and stimulation of astrocytes with carbachol greatly enhances their ability to induce neuritogenesis. This effect is mediated by an effect of carbachol on the expression and release of at least three neuritogenic factors: fibronectin, laminin, and PAI-1 (Guizzetti, et al., 2008). Ethanol can interfere with muscarinic receptor signaling in astrocytes and inhibit their ability to foster neuritogenesis in hippocampal neurons (Guizzetti, et al., 2010). More germane to this project is the finding that manganese, by accumulating and causing oxidative stress in astrocytes, inhibits their ability to induce neuritogenesis in hippocampal neurons (Giordano, et al., 2009).
These initial findings obtained with OPs or with other compounds served as the basis for some of the hypotheses and the Specific Aims indicated above.
The first series of studies sought to investigate whether the widely-used OP diazinon (DZ), and its oxygen metabolite diazoxon (DZO), would affect glial-neuronal interactions as a potential mechanism of developmental neurotoxicity. Specifically, the effects of DZ and DZO on the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons were investigated (Pizzurro, et al., 2014a). The results showed that both DZ and DZO adversely affect astrocyte function, resulting in inhibited neurite outgrowth in hippocampal neurons. This effect appears to be mediated by oxidative stress, as indicated by OP-induced increased reactive oxygen species production in astrocytes and prevention of neurite outgrowth inhibition by antioxidants. The concentrations of OPs were devoid of cytotoxicity, and cause limited acetylcholinesterase inhibition in astrocytes (18 and 25% for DZ and DZO, respectively). Among astrocytic neuritogenic factors, a most important one is the extracellular matrix protein fibronectin. DZ and DZO decreased levels of fibronectin in astrocytes, and this effect also was attenuated by antioxidants. Underscoring the importance of fibronectin in this context, adding exogenous fibronectin to the co-culture system successfully prevented inhibition of neurite outgrowth caused by DZ and DZO.
These results indicate that DZ and DZO increase oxidative stress in astrocytes and this, in turn, modulates astrocytic fibronectin, leading to impaired neurite outgrowth in hippocampal neurons.
A second series of studies focused again on diazinon (DZ) and its active oxygen metabolite, diazoxon (DZO), and explored their ability to directly impair neurite outgrowth in rat primary hippocampal neurons as a mechanism of developmental neurotoxicity (Pizzurro, et al., 2014b). Both DZ and DZO (0.5 - 10 μM) significantly inhibited neurite outgrowth in hippocampal neurons, at concentrations devoid of any cytotoxicity. These effects appeared to be mediated by oxidative stress, as they were prevented by antioxidants (melatonin, N-t-butyl-alpha-phenylnitrone, and glutathione ethyl ester). Inhibition of neurite outgrowth was observed at concentrations below those required to inhibit the catalytic activity of acetylcholinesterase. The presence of astrocytes in the culture was able to provide protection against inhibition of neurite outgrowth by DZ and DZO. Astrocytes increased neuronal glutathione (GSH) in neurons to levels comparable to those of GSH ethyl ester. Astrocytes depleted of GSH by L-buthionine-(S,R)-sulfoximine no longer conferred protection against DZ- and DZO-induced inhibition of neurite outgrowth. The findings indicate that DZ and DZO inhibit neurite outgrowth in hippocampal neurons by mechanisms involving oxidative stress, and that these effects can be modulated by astrocytes and astrocyte-derived GSH. Oxidative stress from other chemical exposures, as well as genetic abnormalities that result in deficiencies in GSH synthesis and regulation, may render individuals more susceptible to these developmental neurotoxic effects of OPs.
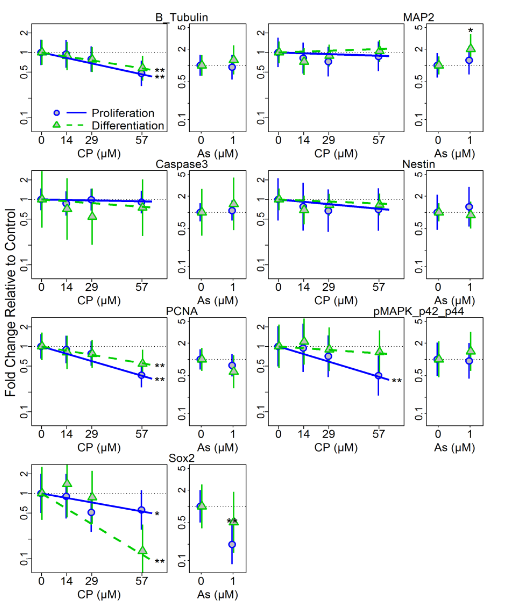
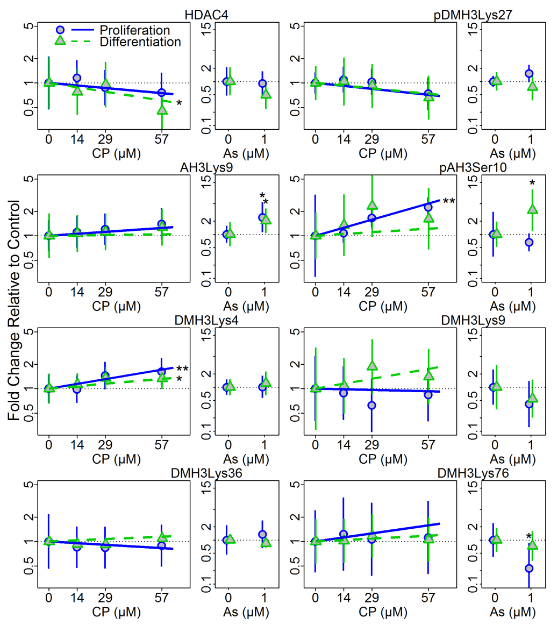
In conjunction with Dr. Costa, Dr. Faustman’s lab also has been examining developmental neurotoxic effects of OPs. In vitro models of neuronal differentiation are emerging as an important tool for high throughput and high content screening in neurodevelopmental toxicology. Understanding when, how, and at what doses neurotoxicants exposures affect normal development is critical for our ability to predict impacts of exposures before population-wide exposures occur. To further explore the importance of developmental context and timing in neurotoxicity, we exposed human neuronal progenitor cells (hNPCs) grown in proliferating and differentiating conditions to chlorpyrifos (CP) and arsenic (As), two well established neurotoxicants. The effects of CP or As treatment on hNPC morphology and cell viability were measured 24 h and 72 h post-treatment and at 72 h post-treatment, changes in protein expression levels of neural differentiation and cell stress markers, cell viability, and histone H3 modifications were observed (Figures 1 and 2). Cell viability, differentiation status, and epigenetic results suggest that hNPC cultures respond to CP and As treatment with different degrees of sensitivity, dependent on differentiation/proliferation status and on the toxicant concentration and length of exposure. Toxicant-related responses in sensitivity and protein expression patterns of neuronal markers that occurred 72 h post-treatment were dependent on the cell growth conditions. Histone modifications, as measured by changes in histone H3 phosphorylation, acetylation, and methylation, varied for each toxicant and growth condition, suggesting that differentiation status can influence the epigenetic effects of CP and As exposures (Figure 2).
Figure 1: Changes in neuronal and stage specific protein marker expressions following treatment of hNPC cultured under proliferation and differentiation conditions. Protein expression was quantified from western blotting following 72h treatment with CP or As under proliferating or differentiating conditions. Plots show means and 95% confidence intervals. * = p<0.05; ** = p <0.01
Figure 2: Changes of histone H3 acetylation and methylation following CP and As treatment of hNPC cultured under proliferation and differentiation conditions at 72 h post treatment. Histone modifications at specific sites were quantified by western blotting following 72h treatment with CP or As under proliferating or differentiating conditions. Plots show means and 95% confidence intervals. * = p<0.05; ** = p<0.01.
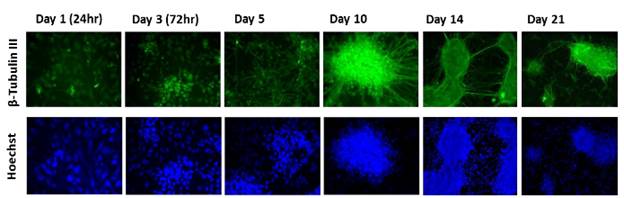
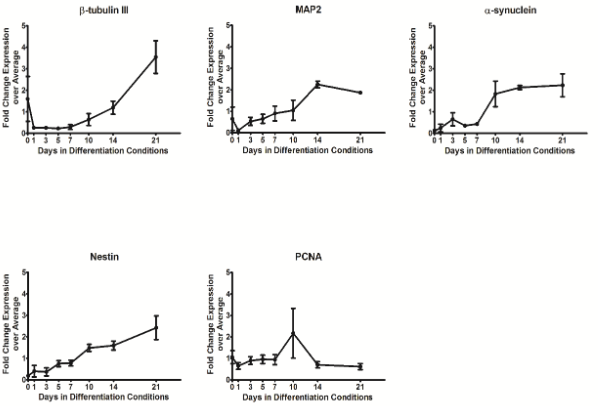
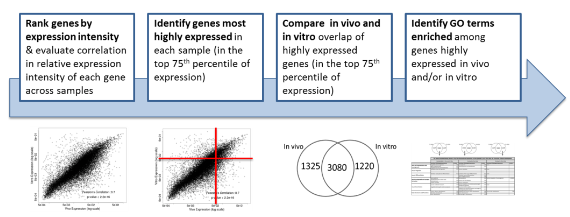
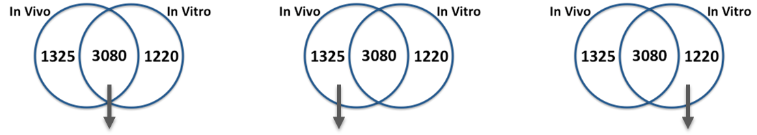
In addition, Dr. Faustman’s lab has characterized pathway dynamics throughout neuronal differentiation of the hNPC line that provides a particularly promising, scalable, and reproducible model for high throughput and high content neurodevelopmental toxicity screening. The lab cultured hNPCs up to 21 days in differentiation conditions and used Western blotting and immunofluorescence to measure changes in protein expression though time (Figures 3 and 4). Global gene expression dynamics were measured using Affymetrix Human gene 2.0 ST microarrays. Over time in differentiation conditions, hNPCs acquired morphological characteristics of mature neuronal networks and increased expression of neuronal differentiation markers, including beta tubulin III, MAP2, and synaptophysin. Significantly changed genes were organized according to temporal expression patterns using K-means clustering, revealing three phases of gene expression. Quantitative pathway analysis identified gene ontology (GO) terms enriched among genes expressed in each of these phases and created a quantitative summary or temporal pathway trends in vitro. GO terms enriched among genes significantly decreased over time are largely associated with proliferation, and stem cell maintenance. GO terms enriched among genes with significantly increasing expression over time are dominated by key developmental processes, including neuronal differentiation, migration, and synaptogenesis. Enrichment of several GO terms associated with forebrain development indicates that these culture conditions promote differentiation towards a forebrain identity. We compared gene expression in vitro with publicly available gene expression data from developing human brain tissue in vivo and found substantial concordance in relative gene expression intensity (Figure 5). Genes highly expressed in both samples were enriched for key processes of brain development, including proliferation, migration, differentiation, synapse formation, and neurotransmission (Table 1). Conversely, GO terms enriched among genes highly expressed only in vivo or only in vitro reveal important differences between systems. For example, genes highly expressed in vitro are enriched for more stress and apoptosis pathways. This analysis provides a timeline of progression through differentiation, facilitating identification of key phases of sensitivity in vitro. Key processes important for the identification of Adverse Outcome Pathways (AOPs) of proliferation, differentiation, and functional maturation matched in vivo patterns (Table 1). Given the heightened sensitivity of the brain to toxicant perturbation during critical windows of development, it is important that we understand which sensitive developmental pathways are captured in vitro and which are not so that in vitro assays can be interpreted appropriately. These observations of morphology, protein, and gene expression provide a timeline of progression through differentiation, facilitating identification of key phases of sensitivity. By anchoring in vitro dynamics to in vivo reference points, this work clarifies the extent to which fundamental processes of brain development are captured in our model.
Figure 3. Morphological development of differentiating hNPCs. Differentiating hNPCs were fixed and β-tubulin III expression was visualized with a fluorescent tag (green). Nuclei are countered stained with Hoechst 33342 (blue). Cells were visualized with a fluorescent microscope at 400x magnification. The increased expression of beta-tubulin III expression and morphological development indicates a growing population of differentiating neurons.
Figure 4. Protein expression in differentiating hNPC cultures through time. Protein was harvested from differentiating hNPCs and expression of specific markers was evaluated by western blotting, with equal amounts of protein loaded in each sample. Data is normalized to Actin expression and presented as fold change expression intensity at each timepoint over expression average across time and reflects results of three independent experiments. Error bars indicate standard error. β-tubulin III, MAP2, α-synuclein, and nestin expression all increase significantly over time (one-way ANOVA p<0.05).
Figure 5. Methods for comparison of gene expression in actively differentiating tissues (in vivo period 2 vs. in vitro day 14)
Table 1. Summary of pathways related to brain development enriched among genes highly expressed (>75th percentile of relative expression intensity) in vivo period 2 vs. in vitro D14
|
 | ||||||
|---|---|---|---|---|---|---|
| GO terms enriched among genes above the 75th percentile of expression In Vivo (period 2) and In Virto (day 14): Processes of brain development | ||||||
| Enriched Both in Vivo and in Vitro | Enriched In Vivo Only | Enriched In Vitro Only | ||||
| enriched GO biological processes | z-score | enriched GO biological processes | z-score | enriched GO biological processes | z-score | |
| Stem Cell Maintenance | glial cell proliferation | 2.34 | neural precursor cell proliferation | 2.29 | none | |
| regulation of stem cell maintenance | 2.24 | |||||
| Neurogenesis | regulation of neurogenesis | 4.81 | neurogenesis | 2.95 | none | |
| neural migration | cerebral cortex cell migration | neuron migration | 2.98 | none | ||
| neural differentiation | forebrain radial glial cell differentiation | 4.14 | cerebral cortex neuron differentiation | 4.23 | none | |
| forebrain neuron differentiation | 3.24 | |||||
| glial cell differentiation | 2.87 | |||||
| neurite outgrowth and synapse formation | axon guidance | 4.94 | axon guidance | 6.06 | positive regulation of axonogenesis | 2.19 |
| peripheral nervous system axon ensheathment | 2.98 | neuron cell-cell adhesion | 5.62 | |||
| myelin assembly | 2.98 | neuron recognition | 4.98 | |||
| neurotransmission | vesicle-mediated transport | 7.78 | positice regulation of excitatory postsynaptic membrane potential | 4.48 | none | |
| regulation of synaptic vesicle exocytosis | 2.86 | neurotransmitter uptake | 3.42 | |||
| regulation of respiratory gaseous exchange by neurological system process | 2.72 | |||||
| brain structures and functions | corpus callosum development | 3.48 | regulation of nervous system development | 4.85 | neural tube development | 2.30 |
| forebrain development | 3.39 | brain development | 4.25 | |||
| bain development | 3.37 | dentate gyrus development | 3.92 | |||
Conclusions:
The focus of the Faustman and Costa laboratories is to understand the potential for, and magnitude of, impacts of pesticides on neurogenesis and gliogenesis. In both these essential neurodevelopmental pathways, the balance between initial proliferation and subsequent specific differentiation is integral for proper neurodevelopment. In this project critical molecular pathways facilitating proliferation and toxicant response are being investigated. Knowledge about the timing and sensitivity of these critical pathways will directly translate into information relevant for establishing conditions promoting environmental and public health safety.
Significance. By anchoring in vitro gene expression dynamics to in vivo dynamics, the analysis above begins to define the appropriate applications of Dr. Costa’s in vitro model of neuronal differentiation for developmental neurotoxicology. The neuronal differentiation model evaluated here captures several essential processes of early brain development in vivo, including neuronal differentiation and development, neuronal migration, synapse formation, and neurotransmission. This model also captures several generic developmental pathways important throughout development. For example, pathway analysis revealed activity in signal transduction pathways and general differentiation and morphogenesis processes that are ubiquitous in development. Detection of perturbation of generic developmental pathways in this model may be able to predict perturbation in a broader set of developmental contexts.
Taken together, Dr. Faustman’s results support the idea that dose, time, and biological context are important factors that need to be considered when developing in vitro models for toxicity testing, interpreting results, and comparing findings across endpoints and platforms.
Key Findings:
- Both diazinon and diazinon-oxon adversely affect astrocyte function, resulting in inhibited neurite outgrowth in hippocampal neurons. Inhibited outgrowth is associated with neurodegenerative diseases.
- Diazinon and diazinon-oxon increase oxidative stress in astrocytes and this, in turn, modulates astrocytic fibronectin, leading to impaired neurite outgrowth in hippocampal neurons.
- Diazinon and diazinon-oxon significantly inhibited neurite outgrowth in hippocampal neurons, at concentrations devoid of cyototoxicity. These effects appeared to be mediated by oxidative stress, as they were prevented by antioxidants (melatonin, N-t-butyl-alpha-phenylnitrone, and glutathione ethyl ester).
- Inhibition of neurite outgrowth was observed at concentrations below those required to inhibit the catalytic activity of acetylcholinesterase. Diazinon and diazinon-oxon inhibit neurite outgrowth in hippocampal neurons by mechanisms involving oxidative stress, and that these effects can be modulated by astrocytes and astrocyte-derived GSH. These responses are similar to those observed following OP exposure.
- Differentiation status (or developmental context) modifies the epigenetic effects of chlorpyrifos and arsenic exposure.
- Characterization of neurodevelopmental toxicants using human neuroprogenitor cells provides a particularly promising, scalable and reproducible model for high throughput and high content neurodevelopmental toxicity screening.
- We have identified common mechanisms of actions for OP pesticides that have informed our analysis of adverse outcome pathways that link OPs to adverse neurodevelopmental outcomes.
Genetic Susceptibility Research Project
The overall goal of the Genetic Susceptibility Research Project was to develop specific biomarkers of exposure to organophosphate (OP) compounds, and to use these biomarkers to explore gene-environment interactions related to genetic variability in the paraoxonase (PON1) gene, particularly with respect to OP exposures that occur during early development.
Specific Aim 1 was to develop and validate immunomagnetic bead (IMB) isolation protocols for the biomarker proteins of interest: butyrylcholinesterase (BChE), acyl peptide hydrolase (APH), and acetylcholinesterase (AChE), and to use a proteomic approach to validate the use of these proteins as biomarkers of OP exposure.
We had demonstrated that a single step IMB protocol will purify BChE from plasma and APH from red cell extracts. The OP adducts to these proteins have been identified. The serine adduct from the active metabolite of tri-cresyl phosphates was found to undergo a unique aging reaction. In vitro inhibition of BChE with the active metabolite of TCP, cyclic saligenin cresyl phosphate (CBDP) generated both a cresyl phosphoserine and an aged phosphoserine. However, in samples from exposed individuals, only the fully aged phosphoserine modification was observed. More recent experiments have shown that AChE does not age in the same way and retains a cresyl group. We had anti-AChE antibodies generated in chicken (Aves Labs, Tigard, OR) based on the sequence differences between human and chicken upstream from the active site serine. We have used these antibodies to develop a protocol for isolation of the human active site tryptic peptide using the chicken antibodies similar to the protocols that we have developed for the rapid isolation of BChE and APH. We currently are using this protocol to examine AChE adduction by OP insecticides as well as the neurotoxins to which children are exposed during flights in commercial aircraft, the triaryl phosphates.
A third biomarker protein, acylpeptide hydrolase – APH, has proven in our mouse in vivo experiments to be very sensitive to CPO exposure and is useful because it has a half-life three-times as long as BChE (33 d vs. 11 d). We have prepared and sent to CDC OP modified and unmodified APH as well as a small quantity of 15N-labeled APH produced in our E. coli expression system to serve as an internal standard when quantifying OP exposures with mass spectrometry.
The immunomagnetic bead/mass spectrometry protocols are sufficiently sensitive to determine exposures from dried blood spots, which are much easier to collect, ship, and archive.
We also added a fourth potentially useful biomarker to our targeted proteins that are modified by OP exposures. While it has been known for many years, there is no carboxylesterase 1 (CES1) in human serum/plasma, nonetheless, we can pull sufficient CES1 out of white cells to analyze for OP adduction; in fact, CES1 also is known as monocyte carboxylesterase. It has the unique property of being modified by non-bioactivated triaryl phosphates. We have shown that recombinant human CES1 is inhibited directly by tri-meta cresyl phosphate, tri-ortho cresyl phosphate, tri-para cresyl phosphate and the two commercial products, used in jet aircraft engine lubricants at levels of ~3% of the oil, Durad 125 and Syn-O-Ad 8484. A study in the United Kingdom showed that triaryl phosphates (TAPs) were detected on 23% of 100 monitored flights in absence of a major fume event (malfunction of engine seals) (Crump, et al., 2011a, Aircraft Cabin Air Sampling Study, Part 1 of the Final Report, Institute of Environment and Health, Cranfield University). A current study by a former Australian pilot has shown that the engine seals are designed to leak. Thus, all of us including small children and the unborn are exposed to small quantities of triaryl phosphates during flights with unknown consequences, highlighting the need for characterizing biomarkers of TAP exposure.
Specific Aims 2 and 3 made use of knockout and humanized mouse models to evaluate interactions among biomarkers of OP sensitivity, exposure, and response during critical stages of development. The experiments in Aim 2 involved chronic exposure of pregnant mice to CP and CPO. The experiments in Aim 3 involve neonatal exposures.
For the experiments characterizing PON1 status as a biomarker of sensitivity to chlorpyrifos (CP) and CPO during gestation, the initial chronic dose-response studies in pregnant and non-pregnant mice demonstrated steep dose-response curves for inhibition of BChE and AChE, with higher doses leading to weight loss and fetal abnormalities. PON1-/- mice were more sensitive than PON1+/+ mice to the effects of chronic CPO exposure. Fetuses were more resistant than dams to CPO inhibition of BChE, and tgHuPON1R192 females were more resistant than females expressing tgHuPON1Q192. In our earlier experiments, dose-response experiments were completed for CPO that involved gestational exposure of mice of all four genotypes (n=10 pregnant dams per treatment group). After timed matings were performed (n=20 females per treatment group), mice with copulatory plugs were exposed transdermally to CPO daily from GD6 to GD17. PON1+/+, tgHuPON1Q192, and tgHuPON1R192 mice were exposed to vehicle (acetone) or to CPO at 0.75 mg/kg/d or 0.85 mg/kg/d. PON1-/- mice were exposed to vehicle alone, or to CPO at 0.50 mg/kg/d or 0.75 mg/kg/d. Dams were sacrificed at GD18, and fetal and maternal tissues were dissected for measurement of enzyme inhibition in different tissues. Additionally, fetal brains were placed in RNAlater and analyzed for gene expression using microarrays. At GD18 there was greater inhibition of BChE in the plasma of fetuses from PON1-/- and tgHuPON1Q192 dams, as compared to the plasma of fetuses from PON1+/+ and tgHuPON1R192 dams. Thus, the PON1 status of the dam clearly influenced CPO toxicity to the fetus. The gene expression analysis showed that gene expression in brains of the fetuses of the PON1-/- mice was more significantly affected than in brains of fetuses of wild type mice. Also, gene expression in the brains fetuses of the PON1R192 mice was less affected than expression in the brains of fetuses of PON1Q192 mice, consistent with the higher efficiency of hydrolysis of CPO by the PON1R192 alloform (Cole et al. 2014). Thus, PON1 status was important in modulating CPO exposure both pre- and postnatally.
Also interesting was the observation that RBC APH showed a better correlation with brain AChE inhibition than the classic biomarker protein BChE, validating our use of APH as an important and useful biomarker protein for OP exposures. Similar results in fetuses were observed when the four mouse strains were exposed to DZO during gestation (Wan-Fen Li, manuscript in preparation).
Significance. These studies have provided information on the consequences of OP exposure during development and on the role of the human PON1192 alloforms in modulating these consequences. The results are relevant to OP sensitivity in humans, because one-half of the U.S. population of Northern European origin is homozygous for the PON1Q192 allele. The experiments examining the effects of maternal PON1 status are providing important information on the relationship of a mother’s PON1 status to her ability to protect a fetus. There is evidence from studies in various populations that children are exposed, either in utero or postnatally, to different OPs. For example, a study in a New York population identified several diethylphosphates (possible metabolites of diazinon or chlorpyrifos, as well as of other OPs) and malathion dicarboxylic acid (a specific metabolite of malathion) in maternal urine (Engel, et al., 2007, Am J Epidemiol 165(12):1397). The study reported behavioral alterations in newborns, which were associated with the presence of dialkyl-phosphates and appeared to be modulated by PON1 status. Our prenatal exposure experiments with dams of different PON1 status has demonstrated that the PON1-/- dams are less well able to protect their fetuses from CPO exposure.
The biomarker project will provide a much better means of quantifying exposures. For example, it is not possible to determine if an individual has been exposed to chlorpyrifos or its breakdown product trichloro-pyridinol. The protein biomarkers are generated only by exposure to active OP compounds. The development of MS protocols for determining the percentage modification of biomarker proteins will provide a much more accurate determination of target protein inhibition. Currently, a baseline level of activity is required to establish a reasonable estimate of the percentage inhibition of an individual’s BChE. The MS protocols directly determine the percentage modification without the need for a baseline activity determination. The MS analysis of modified protein should be superior to analysis of urinary metabolites for estimating exposures, because it is not possible to know whether a metabolite was taken up directly from the environment or was generated in vivo. Modification of biomarker proteins happens only when active pesticide or its oxon is taken up by an individual. The methods that we have developed for MS analysis of OP adducts will be important for the MS Facility at CDC (Rudy Johnson, Director) in identifying OP-related agents of chemical terrorism and cases of pesticide poisoning. The IMB protocols that we have developed require significantly less than 50 μL of plasma and our preliminary experiments with blood spots indicate that the use of blood spot sample collection, shipping, and analysis together with the MS analyses will greatly facilitate exposure studies. It was not possible previously to study and document low level exposures using the inaccurate Ellman activity assay determinations due to both inter- and intra-individual differences in BChE activity levels. The inclusion of APH as a biomarker protein will provide a much longer window in which to evaluate OP exposures and if coupled with analysis of BChE modification together with a series of post-exposure time points should allow the extrapolation back to the time of exposure in cases of acute exposures. Our E. coli protein expression system is allowing us to generate heavy isotope-labeled (15N) recombinant biomarker proteins for use as internal standards in MS analysis of these proteins for our laboratory as well as for CDC’s programs.
The ability to determine whether or not an individual has been exposed to tricresyl phosphate in an airliner cabin as the result of leaky engine seals is very important. Currently, airlines do not inform passengers of any exposures, despite the aircrews often being taken to the hospital at the end of the flight, or in one informative case where the pilot was taken off the airplane on a stretcher and crew members were taken to the hospital in ambulances. Thus, there is very little information on exposure to children to these toxic fume events, which are estimated to occur with a frequency of 0.5 percent to 1 percent of flights. The biomarker research has demonstrated for the first time a physiological response to TCP exposure. Our recent results in analyzing the bioactivation of the meta and para isomers have shown that the active metabolites are different than the CBDP generated from tri-ortho cresyl phosphate. It will be necessary to decorate purified biomarker proteins with the in vitro bioactivated meta and para isomers, which will provide adducts with different masses than previously characterized for the tri-ortho isomers.
The relevance of our research on gene-environment interactions involving PON1 and its polymorphisms extends beyond pesticide toxicity to multiple other PON1 substrates, including lactone-containing drugs and prodrugs, quorum sensing factors used by Pseudomonas aeruginosa for virulence and biofilm formation, and oxidized lipids involved in cardiovascular disease and other diseases. Our development of a modified PON1 status assay may allow for the early identification of males who will go on to develop Parkinson’s disease.
Females have higher levels of mitochondrial PON2 and modulate oxidative stress better than males. This finding helps to explain the lower frequency of some neurological diseases in females and provides an understanding as to why we were able to identify 41% of males with PD (Furlong, et al., manuscript in preparation).
Journal Articles: 178 Displayed | Download in RIS Format
| Other center views: | All 510 publications | 227 publications in selected types | All 178 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Armstrong JL, Fenske RA, Yost MG, Galvin K, Tchong-French M, Yu J. Presence of organophosphorus pesticide oxygen analogs in air samples. Atmospheric Environment 2013;66:145-150. |
R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit |
|
|
Armstrong JL, Fenske RA, Yost MG, Tchong-French M, Yu J. Comparison of polyurethane foam and XAD-2 sampling matrices to measure airborne organophosphorus pesticides and their oxygen analogs in an agricultural community. Chemosphere 2013:92(4):451-457. |
R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit Exit |
|
|
Armstrong JL, Fitzpatrick CF, Loftus CT, Yost MG, Tchong-French M, Karr CJ. Development of a unique multi-contaminant air sampling device for a childhood asthma cohort in an agricultural environment. Environmental Science: Processes & Impacts 2013;15(9):1760-1767. |
R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit |
|
|
Armstrong JL, Dills RL, Yu J, Yost MG, and Fenske RA. A sensitive LC-MS/MS method for measurement of organophosphorus pesticides and their oxygen analogs in air sampling matrices. Journal of Environmental Science and Health, Part B 2014;49(2):102-108. |
R834514 (Final) R834514C002 (Final) |
Exit |
|
|
Armstrong JL, Yost MG, Fenske RA. Development of a passive air sampler to measure airborne organophosphorus pesticides and oxygen analogs in an agricultural community. Chemosphere 2014;111:135-143. |
R834514 (Final) R834514C002 (Final) |
Exit Exit Exit |
|
|
Asselbergs FW, Guo Y, van Iperen EP, Sivapalaratnam S, Tragante V, Lanktree MB, Lange LA, Almoguera B, Appelman YE, Barnard J, Baumert J, Beitelshees AL, Bhangale TR, Chen YD, Gaunt TR, Gong Y, Hopewell JC, Johnson T, Kleber ME, Langaee TY, Li M, Li YR, Liu K, McDonough CW, Meijs MF, Middelberg RP, Musunuru K, Nelson CP, O'Connell JR, Padmanabhan S, Pankow JS, Pankratz N, Rafelt S, Rajagopalan R, Romaine SP, Schork NJ, Shaffer J, Shen H, Smith EN, Tischfield SE, van der Most PJ, van Vliet-Ostaptchouk JV, Verweij N, Volcik KA, Zhang L, Bailey KR, Bailey KM, Bauer F, Boer JM, Braund PS, Burt A, Burton PR, Buxbaum SG, Chen W, Cooper-Dehoff RM, Cupples LA, deJong JS, Delles C, Duggan D, Fornage M, Furlong CE, Glazer N, Gums JG, Hastie C, Holmes MV, Illig T, Kirkland SA, Kivimaki M, Klein R, Klein BE, Kooperberg C, Kottke-Marchant K, Kumari M, LaCroix AZ, Mallela L, Murugesan G, Ordovas J, Ouwehand WH, Post WS, Saxena R, Scharnagl H, Schreiner PJ, Shah T, Shields DC, Shimbo D, Srinivasan SR, Stolk RP, Swerdlow DI, Taylor HA Jr, Topol EJ, Toskala E, van Pelt JL, van Setten J, Yusuf S, Whittaker JC, Zwinderman AH; LifeLines Cohort Study, Anand SS, Balmforth AJ, Berenson GS, Bezzina CR, Boehm BO, Boerwinkle E, Casas JP, Caulfield MJ, Clarke R, Connell JM, Cruickshanks KJ, Davidson KW, Day IN, de Bakker PI, Doevendans PA, Dominiczak AF, Hall AS, Hartman CA, Hengstenberg C, Hillege HL, Hofker MH, Humphries SE, Jarvik GP, Johnson JA, Kaess BM, Kathiresan S, Koenig W, Lawlor DA, März W, Melander O, Mitchell BD, Montgomery GW, Munroe PB, Murray SS, Newhouse SJ, Onland-Moret NC, Poulter N, Psaty B, Redline S, Rich SS, Rotter JI, Schunkert H, Sever P, Shuldiner AR, Silverstein RL, Stanton A, Thorand B, Trip MD, Tsai MY, van der Harst P, van der Schoot E, van der Schouw YT, Verschuren WM, Watkins H, Wilde AA, Wolffenbuttel BH, Whitfield JB, Hovingh GK, Ballantyne CM, Wijmenga C, Reilly MP, Martin NG, Wilson JG, Rader DJ, Samani NJ, Reiner AP, Hegele RA, Kastelein JJ, Hingorani AD, Talmud PJ, Hakonarson H, Elbers CC, Keating BJ, Drenos F. Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. American Journal of Human Genetics 2012;91(5):823-838. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Asselbergs, F.W., Y. Guo, E.P. van Iperen, S. Sivapalaratnam, V. et al. Large-Scale Gene-Centric Meta-analysis across 32 Studies Identifies Multiple Lipid Loci. Am J Hum Genet, 2012. 91(5):p. 823-38. |
R834514C004 (Final) R834798C005 (Final) |
not available |
|
|
Baker PE, Cole TB, Cartwright M, Suzuki SM, Thummel KE, Lin YS, Co AL, Rettie AE, Kim JH, Furlong CE. Identifying safer anti-wear triaryl phosphate additives for jet engine lubricants. Chemico-Biological Interactions 2013;203(1):257-264. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit Exit |
|
|
Bal-Price AK, Coecke S, Costa L, Crofton KM, Fritsche E, Goldberg A, Grandjean P, Lein PJ, Li A, Lucchini R, Mundy WR, Padilla S, Persico AM, Seiler AE, Kreysa J. Advancing the science of developmental neurotoxicity (DNT): testing for better safety evaluation. ALTEX 2012;29(2):202-215. |
R834514 (2013) |
Exit Exit |
|
|
Balk SJ, Council on Environmental Health, Section on Dermatology. Technical report—ultraviolet radiation: a hazard to children and adolescents. Pediatrics 2011;127(3):e791-e817. |
R834514 (2011) R834514 (2012) R834514 (Final) |
Exit Exit |
|
|
Below JE, Earl DL, Shively KM, McMillin MJ, Smith JD, Turner EH, Stephan MJ, Al-Gazali LI, Hertecant JL, Chitayat D, Unger S, Cohn DH, Krakow D, Swanson JM, Faustman EM, Shendure J, Nickerson DA, Bamshad MJ, University of Washington Center for Mendelian Genomics. Whole-genome analysis reveals that mutations in inositol polyphosphate phosphatase-like 1 cause opsismodysplasia. American Journal of Human Genetics 2013;92(1):137-143. |
R834514 (2013) |
Exit Exit |
|
|
Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Luscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. The Journal of Clinical Investigation 2011;121(7):2693-2708. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Buka I, Osornio-Vargas A, Karr C. Melamine food contamination: relevance to Canadian children. Paediatrics and Child Health 2009;14(4):222-224. |
R834514 (2012) R834514 (2013) R834514 (Final) |
|
|
|
Chen J, Zhang XL, Kusumo H, Costa LG, Guizzetti M. 2013. Cholesterol efflux is differentially regulated in neurons and astrocytes:Implications for brain cholesterol homeostasis. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 1831(2):263-275.PMC3534809. |
R834514 (2013) |
Exit |
|
|
Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environmental Health Perspectives 2010;118(2):284-290. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
|
|
|
Cole TB, Jansen K, Park S, Li W-F, Furlong CE, Costa LG. The toxicity of mixtures of specific organophosphate compounds is modulated by paraoxonase 1 status. Advances in Experimental Medicine and Biology 2010;660:47-60. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514C004 (Final) R831709 (2007) |
Exit Exit |
|
|
Cole TB, Beyer RP, Bammler TK, Park SS, Farin FM, Costa LG, Furlong CE. Repeated developmental exposure of mice to chlorpyrifos oxon is associated with paraoxonase 1 (PON1)-modulated effects on cerebellar gene expression. Toxicological Sciences 2011;123(1):155-169. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Cole TB, Giordano G, Co AL, Mohar I, Kavanagh TJ, Costa LG. Behavioral characterization of GCLM-knockout mice, a model for enhanced susceptibility to oxidative stress. Journal of Toxicology 2011;2011:157687 (7 pp.). |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Cole TB, Fisher JC, Burbacher TM, Costa LG, Furlong CE. Neurobehavioral assessment of mice following repeated postnatal exposure to chlorpyrifos-oxon. Neurotoxicology and Teratology. 2012;34(3):311-22. |
R834514 (Final) R834514C004 (Final) R834693 (2015) |
Exit Exit Exit |
|
|
Cole TB, Fisher JC, Burbacher TM, Costa LG, Furlong CE. Neurobehavioral assessment of mice following repeated postnatal exposure to chlorpyrifos-oxon. Neurotoxicology and Teratology 2012;34(3):311-322. |
R834514 (2011) R834514 (2012) R834514 (2013) R834693 (Final) |
Exit Exit Exit |
|
|
Committee on Environmental Health, Committee on Infectious Diseases, Rogan WJ, Brady MT. Drinking water from private wells and risks to children. Pediatrics 2009;123(6):1599-1605. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Committee on Environmental Health, Tester JM. The built environment: designing communities to promote physical activity in children. Pediatrics 2009;123(6):1591-1598. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Committee on Environmental Health, Committee on Substance Abuse, Committee on Adolescence, Committee on Native American Child Health. Tobacco use: a pediatric disease. Pediatrics 2009;124(5):1474-1484. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Coronado GD, Vigoren EM, Griffith WC, Faustman EM, Thompson B. Organophosphate pesticide exposure among pome and non-pome farmworkers:a subgroup analysis of a community randomized trial. Journal of Occupational and Environmental Medicine 2009;51(4):500-509. |
R834514 (2011) R834514 (Final) R834514C001 (Final) R831709 (2007) R832734 (Final) |
Exit |
|
|
Coronado GD, Griffith WC, Vigoren EM, Faustman EM, Thompson B. Where's the dust? Characterizing locations of azinphos-methyl residues in house and vehicle dust among farmworkers with young children. Journal of Occupational and Environmental Hygiene 2010;7(12):663-671. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C001 (Final) |
Exit |
|
|
Coronado GD, Holte S, Vigoren E, Griffith WC, Faustman E, Thompson B. Organophosphate pesticide exposure and residential proximity to nearby fields:evidence for the drift pathway. Journal of Occupational and Environmental Medicine 2011;53(8):884-891. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C001 (Final) |
Exit |
|
|
Coronado GD, Holte SE, Vigoren EM, Griffith WC, Barr DB, Faustman EM, Thompson B. Do workplace and home protective practices protect farm workers? Findings from the "For Healthy Kids" study. Journal of Occupational and Environmental Medicine 2012;54(9):1163-1169. |
R834514 (2013) R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Costa LG, Giordano G, Guizzetti M. In vitro models to study cell-cell interactions that influence developmental neurotoxicity. ALTEX 2010;27(Special Issue 1):303-308. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Costa LG, Giordano G, Furlong CE. Pharmacological and dietary modulators of paraxonase 1 (PON1) activity and expression: the hunt goes on. Biochemical Pharmacology 2011;81(3):337-344. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology 2013;307:115-122. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Costa LG. The birth and early years of INA, the International Neurotoxicology Association. Neurotoxicology 2013;36:89-103. |
R834514 (2013) |
Exit Exit |
|
|
Costa LG, Giordano G, Guizzetti M. Inhibition of cholinergic muscarinic signaling by ethanol: potential mechanism of developmental neurotoxicity and biological plausibility for the beneficial effects of choline supplementation. The International Journal of Alcohol and Drug Research 2013;2(3):17-25. |
R834514 (2013) |
Exit Exit |
|
|
Costa LG, Pellacani C, Dao K, Kavanagh TJ, Roque PJ. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. NeuroToxicology 2015;48:68-76. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Council on Environmental Health, Section on Dermatology. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics 2011;127(3):588-597. |
R834514 (2013) |
Exit Exit Exit |
|
|
Council on Environmental Health. Chemical-management policy: prioritizing children’s health. Pediatrics 2011;127(5):983-990. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Council on Environmental Health. Policy statement: pesticide exposure in children. Pediatrics 2012;130(6):e1757-e1763. |
R834514 (2012) |
Exit Exit |
|
|
Daniell WE, Van Tung L, Wallace RM, Havens DJ, Karr CJ, Bich Diep N, Croteau GA, Beaudet NJ, Duy Bao N. Childhood lead exposure from battery recyling in Vietnam. Biomed Research International 2015;2015:193715. |
R834514 (Final) |
Exit |
|
|
Furlong CE, Suzuki SM, Stevens RC, Marsillach J, Richter RJ, Jarvik GP, Checkoway H, Samii A, Costa LG, Griffith A, Roberts JW, Yearout D, Zabetian CP. Human PON1, a biomarker of risk of disease and exposure. Chemico-Biological Interactions 2010;187(1-3):355-361. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) |
Exit Exit Exit |
|
|
Furlong CE. Exposure to triaryl phosphates: metabolism and biomarkers of exposure. Journal of Biological Physics and Chemistry 2011;11(4):165-171. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Furlong CE, Marsillach J, Jarvik GP, Costa LG. Paraoxonases-1,-2 and-3: what are their functions? Chemico-Biological Interactions 2016;259(Pt B):51-62. |
R834514 (Final) |
Exit Exit Exit |
|
|
Garrick JM, Dao K, de Laat R, Elsworth J, Cole TB, Marsillach J, Furlong CE, Costa LG. Developmental expression of paraoxonase 2. Chemico-Biological Interactions 2016;259(Pt B):168-174. |
R834514 (Final) |
Exit Exit Exit |
|
|
Gibbs JL, Yost MG, Negrete M, Fenske RA. Passive sampling for indoor and outdoor exposures to chlorpyrifos, azinphos-methyl, and oxygen analogs in a rural agricultural community. Environmental Health Perspectives 2017;125(3):333-341. |
R834514 (Final) R834514C002 (Final) |
|
|
|
Giordano G, Guizzetti M, Dao K, Mattison HA, Costa LG. Ethanol impairs muscarinic receptor-induced neuritogenesis in rat hippocampal slices: role of astrocytes and extracellular matrix proteins. Biochemical Pharmacology 2011;82(11):1792-1799. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Giordano G, Cole TB, Furlong CE, Costa LG. Paraoxonase 2 (PON2) in the mouse central nervous system: a neuroprotective role? Toxicology and Applied Pharmacology 2011;256(3):369-378. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Giordano G, Costa LG. Developmental neurotoxicity: some old and new issues. ISRN Toxicology 2012;2012:814795. |
R834514 (2013) |
Exit Exit |
|
|
Giordano G, Kavanagh TJ, Faustman EM, White CC, Costa LG. Low-level domoic acid protects mouse cerebellar granule neurons from acute neurotoxicity: role of glutathione. Toxicological Sciences 2013;132(2):399-408. |
R834514 (2013) |
Exit Exit Exit |
|
|
Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, Costa LG. Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radical Biology and Medicine 2013;58:98-108. |
R834514 (2013) R834514C004 (Final) |
Exit Exit |
|
|
Griffith W, Curl CL, Fenske RA, Lu CA, Vigoren EM, Faustman EM. Organophosphate pesticide metabolite levels in pre-school children in an agricultural community:within-and between-child variability in a longitudinal study. Environmental Research 2011;111(6):751-756. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit Exit |
|
|
Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia 2010;58(12):1395-1406. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) |
Exit |
|
|
Guizzetti M, Moore NH, VanDeMark KL, Giordano G, Costa LG. Muscarinic receptor-activated signal transduction pathways involved in the neuritogenic effect of astrocytes in hippocampal neurons. European Journal of Pharmacology 2011;659(2-3):102-107. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Guo Y, Lanktree MB, Taylor KC, Hakonsarson H, Lange LA, Keating BJ, IBC 50K SNP Array BMI Consortium. Gene-centric meta-analyses of 108 912 individuals confirm known body mass index loci and reveal three novel signals. Human Molecular Genetics 2013;22(1):184-201. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Harris S, Hermsen SA, Yu X, Hong SW, Faustman EM. Comparison of toxicogenomic responses to phthalate ester exposure in an organotypic testis co-culture model and responses observed in vivo. Reproductive Toxicology 2015;58:149-159. |
R834514 (Final) R834514C003 (2015) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2017) |
Exit Exit Exit |
|
|
Harris S, Wegner S, Hong SW, Faustman EM. Phthalate metabolism and kinetics in an in vitro model of testis development. Toxicology in Vitro 2016;32:123-131. |
R834514 (Final) R834514C003 (Final) R833772 (2009) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2016) R835738C004 (2017) |
Exit Exit Exit |
|
|
Harris S, Shubin SP, Wegner S, Van Ness K, Green F, Hong SW, Faustman EM. The presence of macrophages and inflammatory responses in an in vitro testicular co-culture model of male reproductive development enhance relevance to in vivo conditions. Toxicology In Vitro 2016;36:210-215. |
R834514 (Final) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2016) R835738C004 (2017) |
Exit Exit |
|
|
Hofmann JN, Keifer MC, Furlong CE, De Roos AJ, Farin FM, Fenske RA, van Belle G, Checkoway H. Serum cholinesterase inhibition in relation to paraoxonase-1 (PON1) status among organophosphate-exposed agricultural pesticide handlers. Environmental Health Perspectives 2009;117(9):1402-1408. |
R834514 (2012) R834514 (2013) |
|
|
|
Hofmann JN, Keifer MC, De Roos AJ, Fenske RA, Furlong CE, van Belle G, Checkoway H. Occupational determinants of serum cholinesterase inhibition among organophosphate-exposed agricultural pesticide handlers in Washington State. Occupational and Environmental Medicine 2010;67(6):375-386. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Hohl SD, Gonzalez C, Carosso E, Ibarra G, Thompson B. "I did it for us and I would do it again": perspectives of rural Latinos on providing biospecimens for research. American Journal of Public Health 2014;104(5):911-916. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Holme F, Thompson B, Holte S, Vigoren EM, Espinoza N, Ulrich A, Griffith W, Faustman EM. The role of diet in children's exposure to organophosphate pesticides. Environmental Research 2016;147:133-140. |
R834514 (Final) R834514C001 (2015) R834514C001 (Final) |
Exit Exit Exit |
|
|
Inoue S, Becker AL, Kim J-H, Shu Z, Soelberg SD, Weigel KM, Hiraiwa M, Cairns A, Lee H-B, Furlong CE, Oh K, Lee K-H, Gao D, Chung J-H, Cangelosi GA. Semi-automated, occupationally safe immunofluorescence microtip sensor for rapid detection of mycobacterium cells in sputum. PLoS One 2014;9(1):e86018. |
R834514 (Final) R834514C004 (2015) |
Exit Exit |
|
|
Jackson JE, Yost MG, Karr CJ, Fitzpatrick C, Lamb BK, Chung S, Chen J, Avise J, Rosenblatt RA, Fenske RA. Public health impacts of climate change in Washington State: projected mortality risks due to heat events and air pollution. Climatic Change 2010;102(1-2):159-186. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit |
|
|
Jansen KL, Cole TB, Park SS, Furlong CE, Costa LG. Paraoxonase 1 (PON1) modulates the toxicity of mixed organophosphorus compounds. Toxicology and Applied Pharmacology 2009;236(2):142-153. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Karr CJ, Rudra CB, Miller KA, Gould TR, Larson T, Sathyanarayana S, Koenig JQ. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environmental Research 2009;109(3):321-327. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Karr CJ, Demers PA, Koehoorn MW, Lencar CC, Tamburic L, Brauer M. Influence of ambient air pollutant sources on clinical encounters for infant bronchiolitis. American Journal of Respiratory and Critical Care Medicine 2009;180(10):995-1001. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Karr CJ. Adding fuel to the fire: increasing evidence for developmental toxicity of indoor solid fuel combustion. Archives of Pediatric & Adolescent Medicine 2011;165(6):565-566. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Karr CJ. Children's environmental health in agricultural settings. Journal of Agromedicine 2012;17(2):127-139. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Karr C. Addressing environmental contaminants in pediatric practice. Pediatrics in Review 2011;32(5):190-200. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Richter RJ, Marshall JK, Eintracht JF, Rosenthal EA, Furlong CE, Jarvik GP. Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. Journal of Lipids 2012;2012:476316 (11 pp.). |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Richter RJ, Marshall JK, Nakayama KS, Jarvik ER, Eintracht JF, Rosenthal EA, Furlong CE, Jarvik JP. Dietary cholesterol increases paraoxonase 1 enzyme activity. Journal of Lipid Research 2012;53(11):2450-2458. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Kim DS, Burt AA, Crosslin DR, Robertson PD, Ranchalis JE, Boyko EJ, Nickerson DA, Furlong CE, Jarvik GP. Novel common and rare genetic determinants of paraoxonase activity: FTO, SERPINA12, and ITGAL. Journal of Lipid Research 2013;54(2):552-560. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Jarvik ER, Rosenthal EA, Hatsukami TS, Furlong CE, Jarvik GP. Novel gene-by-environment interactions: APOB and NPC1L1 variants affect the relationship between dietary and total plasma cholesterol. Journal of Lipid Research 2013;54(5):1512-1520. |
R834514 (2013) |
Exit Exit Exit |
|
|
Kim DS, Marsillach J, Furlong CE, Jarvik GP. Pharmacogenetics of paraoxonase activity: elucidating the role of high-density lipoprotein in disease. Pharmacogenomics 2013;14(12):1495-1515. |
R834514 (2013) R834514C004 (Final) |
Exit |
|
|
Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, Eintracht JF, Hatsukami TS, Furlong CE, Marcovina S, Albers JJ, Jarvik GP. HDL-3 is a superior predictor of carotid artery disease in a case-control cohort of 1725 participants. Journal of the American Heart Association 2014;3(3):e000902. |
R834514 (Final) R834514C004 (2015) |
Exit Exit |
|
|
Kim DS, Crosslin DR, Auer PL, Suzuki SM, Marsillach J, Burt AA, Gordon AS, Meschia JF, Nalls MA, Worrall BB, Longstreth Jr. WT, Gottesman RF, Furlong CE, Peters U, Rich SS, Nickerson DA, Jarvik GP. Rare coding variation in paraoxonase-1 is associated with ischemic stroke in the NHLBI Exome Sequencing Project. Journal of Lipid Research 2014;55(6):1173-1178. |
R834514 (Final) R834514C004 (2015) |
Exit Exit Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Jarvik LE, Eintracht JF, Furlong CE, Jarvik GP. Effects of dietary components on high-density lipoprotein measures in a cohort of 1,566 participants. Nutrition & Metabolism 2014;11(1):44 (9 pp.). |
R834514C004 (2015) |
Exit Exit |
|
|
Kim DS, Burt AA, Ranchalis JE, Vuletic S, Vaisar T, Li WF, Rosenthal EA, Dong W, Eintracht JF, Motulsky AG, Brunzell JD, Albers JJ, Furlong CE, Jarvik GP. PLTP activity inversely correlates with CAAD: effects of PON1 enzyme activity and genetic variants on PLTP activity. Journal of Lipid Research 2015;56(7):1351-1362. |
R834514 (Final) R834514C004 (2015) |
Exit Exit Exit |
|
|
Kim DS, Li YK, Bell GA, Burt AA, Vaisar T, Hutchins PM, Furlong CE, Otvos JD, Polak JF, Arnan MK, Kaufman JD, McClelland RL, Longstreth Jr WT, Jarvik GP. Concentration of smaller high-density lipoprotein particle (HDL-P) is inversely correlated with carotid intima media thickening after confounder adjustment: the Multi Ethnic Study of Atherosclerosis (MESA). Journal of the American Heart Association 2016;5(5):e002977. |
R834514 (Final) |
Exit Exit Exit |
|
|
Kim HY, Wegner SH, Van Ness KP, Park JJ, Pacheco SE, Workman T, Hong S, Griffith W, Faustman EM. Differential epigenetic effects of chlorpyrifos and arsenic in proliferating and differentiating human neural progenitor cells. Reproductive Toxicology 2016;65:212-223. |
R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Kim JH, Stevens RC, MacCoss MJ, Goodlett DR, Scherl A, Richter RJ, Suzuki SM, Furlong CE. Identification and characterization of biomarkers of organophosphorus exposures in humans. Advances in Experimental Medicine and Biology 2010;660:61-71. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Kim JH, Yeo WH, Shu Z, Soelberg SD, Inoue S, Kalyanasundaram D, Ludwig J, Furlong CE, Riley JJ, Weigel KM, Cangelosi GA, Oh K, Lee KH, Gao D, Chung JH. Immunosensor towards low-cost, rapid diagnosis of tuberculosis. Lab on a Chip 2012;12(8):1437-1440. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Kim NJ, Vasquez VB, Torres E, Nicola RM, Karr C. Breaking the silence: sexual harassment of Mexican women farmworkers. Journal of Agromedicine 2016;21(2):154-162. |
R834514 (Final) |
Exit |
|
|
Kullman SW, Hamm JT, Hinton DE. Identification and characterization of a cDNA encoding cytochrome P450 3A from the fresh water teleost medaka (Oryzias latipes). Archives of Biochemistry and Biophysics 2000;380(1):29-38. |
R834514 (2011) R834514 (Final) R825298 (Final) |
Exit Exit |
|
|
Lanktree MB, Guo Y, Murtaza M, Glessner JT, Bailey SD, Onland-Moret NC, Lettre G, Ongen H, Rajagopalan R, Johnson T, Shen H, Nelson CP, Klopp N, Baumert J, Padmanabhan S, Pankratz N, Pankow JS, Shah S, Taylor K, Barnard J, Peters BJ, Maloney CM, Lobmeyer MT, Stanton A, Zafarmand MH, Romaine SP, Mehta A, van Iperen EP, Gong Y, Price TS, Smith EN, Kim CE, Li YR, Asselbergs FW, Atwood LD, Bailey KM, Bhatt D, Bauer F, Behr ER, Bhangale T, Boer JM, Boehm BO, Bradfield JP, Brown M, Braund PS, et al. Meta-analysis of dense genecentric association studies reveals common and uncommon variants associated with height. American Journal of Human Genetics 2011;88(1):6-18. |
R834514 (2011) R834514 (Final) |
Exit Exit |
|
|
Liyasova M, Li B, Schopfer LM, Nachon F, Masson P, Furlong CE, Lockridge O. Exposure to tri-o-cresyl phosphate detected in jet airplane passengers. Toxicology and Applied Pharmacology 2011;256(3):337-347. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Loftus C, Yost M, Sampson P, Arias G, Torres E, Vasquez VB, Bhatti P, Karr C. Regional PM2.5 and asthma morbidity in an agricultural community: a panel study. Environmental Research 2015;136:505-512. |
R834514 (2015) R834514 (Final) R834514C002 (2015) R834514C002 (Final) |
Exit Exit Exit |
|
|
Loftus C, Yost M, Sampson P, Torres E, Arias G, Breckwich Vasquez V, Hartin K, Armstrong J, Tchong French M, Vedal S, Bhatti P, Karr C. Ambient ammonia exposures in an agricultural community and pediatric asthma morbidity. Epidemiology 2015;26(6):794-801. |
R834514 (2015) |
Exit |
|
|
MacIntyre EA, Karr CJ, Koehoorn M, Demers P, Tamburic L, Lencar C, Brauer M. Otitis media incidence and risk factors in a population-based birth cohort. Paediatrics and Child Health 2010;15(7):437-442. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
MacIntyre EA, Karr CJ, Koehoorn M, Demers PA, Tamburic L, Lencar C, Brauer M. Residential air pollution and otitis media during the first two years of life. Epidemiology 2011;22(1):81-89. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicology and Teratology 2010;32(4):452-459. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Malen R, Knerr S, Delgado F, Fullerton SM, Thompson B. Rural Mexican-Americans’ perceptions of family health history, genetics, and disease risk: implications for disparities-focused research dissemination. Journal of Community Genetics 2016;7(1):91-96. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Marsillach J, Richter RJ, Kim JH, Stevens RC, MacCoss MJ, Tomazela D, Suzuki SM, Schopfer LM, Lockridge O, Furlong CE. Biomarkers of organophosphorus (OP) exposures in humans. NeuroToxicology 2011;32(5):656-660. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Marsillach J, Hsieh EJ, Richter RJ, MacCoss MJ, Furlong CE. Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chemico-Biological Interactions 2013;203(1):85-90. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Marsillach J, Costa LG, Furlong CE. Protein adducts as biomarkers of exposure to organophosphorus compounds. Toxicology 2013;307:46-54. |
R834514 (2013) R834514C004 (Final) |
Exit Exit |
|
|
Marsillach J, Suzuki SM, Richter RJ, McDonald MG, Rademacher PM, MacCoss MJ, Hsieh EJ, Rettie AE, Furlong CE. Human valacyclovir hydrolase/biphenyl hydrolase-like protein is a highly efficient homocysteine thiolactonase. PLoS One 2014;9(10):e110054. |
R834514 (Final) R834514C004 (2015) |
Exit Exit |
|
|
Marsillach J, Becker JO, Vaisar T, Hahn BH, Brunzell JD, Furlong CE, deBoer IH, McMahon MA, Hoofnagle AN, DCCT/EDIC Research Group. Paraoxonase-3 is depleted from the high-density lipoproteins of autoimmune disease patients with subclinical atherosclerosis. Journal of Proteome Research 2015;14(5):2046-2054. |
R834514 (Final) R834514C004 (2015) |
Exit Exit Exit |
|
|
Marsillach J, Costa LG, Furlong CE. Paraoxonase-1 and early-life environmental exposures. Annals of Global Health 2016;82(1):100-110. |
R834514 (Final) |
Exit Exit Exit |
|
|
McMillin MJ, Below JE, Shively KM, Beck AE, Gildersleeve HI, Pinner J, Gogola GR, Hecht JT, Grange DK, Harris DJ, Earl DL, Jagadeesh S, Mehta SG, Robertson SP, Swanson JM, Faustman EM, Mefford HC, Shendure J, Nickerson DA, Bamshad MJ, University of Washington Center for Mendelian Genomics. Mutations in ECEL1 cause distal arthrogryposis type 5D. American Journal of Human Genetics 2013;92(1):150-156. |
R834514 (2013) |
Exit Exit |
|
|
Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, Vokonas PS, Schwartz JD. Long-term exposure to ambient fine particulate matter and renal function in older men: the Veterans Administration Normative Aging Study. Environmental Health Perspectives 2016;124(9):1353-1360. |
R834514C004 (Final) R834798 (Final) R834798C002 (Final) |
|
|
|
Moreira EG, Yu X., Robinson JF, Griffith W, Hong SW, Beyer RP, Bammler TK, Faustman EM. Toxicogenomic profiling in maternal and fetal rodent brains following gestational exposure to chlorpyrifos. Toxicology and Applied Pharmacology 2010;245(3):310-325. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (2007) |
Exit Exit Exit |
|
|
Nonnenmann MW, Coronado G, Thompson B, Griffith WC, Hanson JD, Vesper S, Faustman EM. Utilizing pyrosequencing and quantitative PCR to characterize fungal populations among house dust samples. Journal of Environmental Monitoring 2012;14(8):2038-2043. |
R834514 (2013) R834514 (Final) |
Exit |
|
|
O’Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Reviews on Environmental Health 2008;23(3):167-202. |
R834514 (2011) R834514 (Final) EM833367 (2009) EM833367 (Final) |
Exit |
|
|
Paulson JA, Karr CJ, Seltzer JM, Cherry DC, Sheffield PE, Cifuentes E, Buka I, Amler RW. Development of the pediatric environmental health specialty unit network in North America. American Journal of Public Health 2009;99(Suppl 3):S511-S516. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Perkins A, Walters F, Sievert J, Rhodes B, Morrissey B, Karr CJ. Home use of a pyrethroid-containing pesticide and facial paresthesia in a toddler:a case report. International Journal of Environmental Research and Public Health 2016;13(8):829 (7 pp.). |
R834514 (Final) |
Exit Exit Exit |
|
|
Perla ME, Rue T, Cheadle A, Krieger J, Karr CJ. Population-based comparison of biomarker concentrations for chemicals of concern among Latino-American and non-Hispanic white children. Journal of Immigrant and Minority Health 2015;17(3):802-819. |
R834514 (2015) |
Exit |
|
|
Perla ME, Iman E, Campos L, Perkins A, Liebman AK, Miller ME, Beaudet NJ, Karr CJ. Agricultural occupational health and safety perspectives among Latino-American youth. Journal of Agromedicine 2015;20(2):167-177. |
R834514 (2015) |
|
|
|
Perla ME, Rue T, Cheadle A, Krieger J, Karr CJ. Biomarkers of insecticide exposure and asthma in children: a National Health and Nutrition Examination Survey (NHANES) 1999-2008 analysis. Archives of Environmental & Occupational Health 2015;70(6):309-322. |
R834514 (2015) |
Exit |
|
|
Pizzurro DM, Dao K, Costa LG. Astrocytes protect against diazinon- and diazoxon-induced inhibition of neurite outgrowth by regulating neuronal glutathione. Toxicology 2014;318:59-68. |
R834514C003 (Final) |
Exit Exit Exit |
|
|
Pizzurro DM, Dao K, Costa LG. Diazinon and diazoxon impair the ability of astrocytes to foster neurite outgrowth in primary hippocampal neurons. Toxicology and Applied Pharmacology 2014;274(3):372-382. |
R834514C003 (Final) |
Exit Exit Exit |
|
|
Plascak JJ, Molina Y, Wu-Georges S, Idris A, Thompson B. Latino residential segregation and self-rated health among Latinos: Washington State Behavioral Risk Factor Surveillance System, 2012-2014. Social Science & Medicine 2016;159:38-47. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Postma J, Karr C, Kieckhefer G. Community health workers and environmental interventions for children with asthma: a systematic review. Journal of Asthma 2009;46(6):564-576. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Postma J, Younglove LR, Odom-Maryon T, Brooks K, Burbacher TM, Butterfield PW, Butterfield PG, Cederblom N, Grant K, Beresford SAA, Faustman EM. Hispanic representation in a longitudinal birth cohort study. Journal of Health Disparities Research and Practice 2016;9(2):66-77. |
R834514 (Final) |
Exit Exit |
|
|
Raanan R, Gunier RB, Balmes JR, Beltran AJ, Harley KG, Bradman A, Eskenazi B. Elemental sulfur use and associations with pediatric lung function and respiratory symptoms in an agricultural community (California, USA). Environmental Health Perspectives 2017;125(8):087007 (8 pp.). |
R834514 (Final) R826709 (2002) R831710 (Final) R834513 (Final) |
|
|
|
Ramaprasad J, Tsai MG, Fenske RA, Faustman EM, Griffith WC, Felsot AS, Elgethun K, Weppner S, Yost MG. Children's inhalation exposure to methamidophos from sprayed potato fields in Washington State:exploring the use of probabilistic modeling of meteorological data in exposure assessment. Journal of Exposure Science and Environmental Epidemiology 2009;19(6):613-623. |
R834514 (Final) R834514C002 (Final) R831709 (2007) |
Exit Exit |
|
|
Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicology and Applied Pharmacology 2009;235(1):1-9. |
R834514 (2012) R834514 (2013) R834514 (Final) R831709 (2007) |
Exit Exit Exit |
|
|
Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 status as a risk factor for disease or exposure. Advances in Experimental Medicine and Biology 2010;660:29-35. |
R834514 (2011) R834514 (2013) |
Exit |
|
|
Roberts JR, Karr CJ, Council on Environmental Health. Pesticide exposure in children. Pediatrics 2012;130(6):e1765-e1788. |
R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Roberts JR, Karr CJ, de Ybarrondo L, McCurdy LE, Freeland KD, Husley TC, Forman J. Improving pediatrician knowledge about environmental triggers of asthma. Clinical Pediatrics 2013;52(6):527-533. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Roberts JR, Newman N, McCurdy LE, Chang JS, Salas MA, Eskridge B, De Ybarrondo L, Sandel M, Mazur L, Karr CJ. Integrating environmental management of asthma into pediatric health care: what worked and what still needs improvement? Clinical Pediatrics 2016;55(14):1271-1278. |
R834514 (Final) |
Exit Exit |
|
|
Robinson JF, Guerrette Z, Yu X, Hong S, Faustman EM. A systems-based approach to investigate dose-and time-dependent methylmercury-induced gene expression response in C57BL/6 mouse embryos undergoing neurulation. Birth Defects Research. Part B: Developmental and Reproductive Toxicology 2010;89(3):188-200. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) |
Exit |
|
|
Robinson JF, Yu X, Hong S, Zhou C, Kim N, DeMasi D, Faustman EM. Embryonic toxicokinetic and dynamic differences underlying strain sensitivity to cadmium during neurulation. Reproductive Toxicology 2010;29(3):279-285. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) |
Exit Exit Exit |
|
|
Robinson JF, Port JA, Yu X, Faustman EM. Integrating genetic and toxicogenomic information for determining underlying susceptibility to developmental disorders. Birth Defects Research. Part A--Clinical and Molecular Teratology 2010;88(10):920-930. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Robinson JF, Griffith WC, Yu X, Hong S, Kim E, Faustman EM. Methylmercury induced toxicogenomic response in C57 and SWV mouse embryos undergoing neural tube closure. Reproductive Toxicology 2010;30(2):284-291. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Yu X, Moreira EG, Hong S, Faustman EM. Arsenic-and cadmium-induced toxicogenomic response in mouse embryos undergoing neurulation. Toxicology and Applied Pharmacology 2011;250(2):117-129. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) |
Exit Exit Exit |
|
|
Robinson JF, Theunissen PT, van Dartel DA, Pennings JL, Faustman EM, Piersma AH. Comparison of MeHg-induced toxicogenomic responses across in vivo and in vitro models used in developmental toxicology. Reproductive Toxicology 2011;32(2):180-188. |
R834514 (Final) R834514C003 (Final) |
Exit Exit Exit |
|
|
Sathyanarayana S, Basso O, Karr CJ, Lozano P, Alavanja M, Sandler DP, Hoppin JA. Maternal pesticide use and birth weight in the Agricultural Health Study. Journal of Agromedicine 2010;15(2):127-136. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Sathyanarayana S, Zhou C, Rudra CB, Gould T, Larson T, Koenig J, Karr CJ. Prenatal ambient air pollution exposure and small for gestational age birth in the Puget Sound Air Basin. Air Quality, Atmosphere & Health 2013;6(2):455-463. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Saxena R, Elbers CC, Guo Y, Peter I, Gaunt TR, Mega JL, Lanktree MB, Tare A, Castillo BA, Li YR, Johnson T, Bruinenberg M, Gilbert-Diamond D, Rajagopalan R, Voight BF, Balasubramanyam A, Barnard J, Bauer F, Baumert J, Bhangale T, Bohm BO, Braund PS, Burton PR, Chandrupatla HR, Clarke R, Cooper-DeHoff RM, Crook ED, Davey-Smith G, Day IN, de Boer A, de Groot MC, Drenos F, Ferguson J, Fox CS, Furlong CE, Gibson Q, Gieger C, Gilhuijs-Pederson LA, Glessner JT, Goel A, Gong Y, Grant SF, Grobbee DE, Hastie C, Humphries SE, Kim CE, Kivimaki M, Kleber M, Meisinger C, Kumari M, Langaee TY, Lawlor DA, Li M, Lobmeyer MT, Maitland-van der Zee AH, Meijs MF, Molony CM, Morrow DA, Murugesan G, Musani SK, Nelson CP, Newhouse SJ, O'Connell JR, Padmanabhan S, Palmen J, Patel SR, Pepine CJ, Pettinger M, Price TS, Rafelt S, Ranchalis J, Rasheed A, Rosenthal E, Ruczinski I, Shah S, Shen H, Silbernagel G, Smith EN, Spijkerman AW, Stanton A, Steffes MW, Thorand B, Trip M, van der Harst P, van der A DL, van Iperen EP, van Setten J, van Vliet-Ostaptchouk JV, Verweij N, Wolffenbuttel BH, Young T, Zafarmand MH, Zmuda JM; Look AHEAD Research Group; DIAGRAM consortium, Boehnke M, Altshuler D, McCarthy M, Kao WH, Pankow JS, Cappola TP, Sever P, Poulter N, Caulfield M, Dominiczak A, Shields DC, Bhatt DL, Zhang L, Curtis SP, Danesh J, Casas JP, van der Schouw YT, Onland-Moret NC, Doevendans PA, Dorn GW 2nd, Farrall M, FitzGerald GA, Hamsten A, Hegele R, Hingorani AD, Hofker MH, Huggins GS, Illig T, Jarvik GP, Johnson JA, Klungel OH, Knowler WC, Koenig W, März W, Meigs JB, Melander O, Munroe PB, Mitchell BD, Bielinski SJ, Rader DJ, Reilly MP, Rich SS, Rotter JI, Saleheen D, Samani NJ, Schadt EE, Shuldiner AR, Silverstein R, Kottke-Marchant K, Talmud PJ, Watkins H, Asselbergs FW, de Bakker PI, McCaffery J, Wijmenga C, Sabatine MS, Wilson JG, Reiner A, Bowden DW, Hakonarson H, Siscovick DS, Keating BJ. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. American Journal of Human Genetics 2012;90(3):410-425. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Schopfer LM, Furlong CE, Lockridge O. Development of diagnostics in the search of an explanation of aerotoxic syndrome. Analytical Biochemistry 2010;404(1):64-74. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Serrano SE, Karr CJ, Seixas NS, Nguyen RH, Barrett ES, Janssen S, Redmon B, Swan SH, Sathyanarayana S. Dietary phthalate exposure in pregnant women and the impact of consumer practices. International Journal of Environmental Research and Public Health 2014;11(6):6193-6215. PMID:24927036. |
R834514 (2015) |
Exit Exit |
|
|
Shepherd-Banigan M, Ulrich A, Thompson B. Macro-activity patterns of farmworker and non-farmworker children living in an agricultural community. Environmental Research 2014;132:176-181. |
R834514 (Final) R834514C001 (Final) |
Exit Exit Exit |
|
|
Shepherd-Banigan M, Hohl SD, Vaughan C, Ibarra G, Carosso E, Thompson B. "The promotora explained everything": participant experiences during a household-level diabetes education program. The Diabetes Educator 2014;40(4):507-515. |
R834514C001 (2015) |
Exit |
|
|
Smith MN, Griffith WC, Beresford SA, Vredevoogd M, Vigoren EM, Faustman EM. Using a biokinetic model to quantify and optimize cortisol measurements for acute and chronic environmental stress exposure during pregnancy. Journal of Exposure Science and Environmental Epidemiology 2014;24(5):510-516. |
R834514 (2013) R834514 (Final) |
Exit |
|
|
Smith MN, Wilder CS, Griffith WC, Workman T, Thompson B, Dills R, Onstad G, Vredevoogd M, Vigoren EM, Faustman EM. Seasonal variation in cortisol biomarkers in Hispanic mothers living in an agricultural region. Biomarkers 2015;20(5):299-305. |
R834514 (2015) R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Smith MN, Grice J, Cullen A, Faustman EM. A toxicological framework for the prioritization of Children’s Safe Product Act data. International Journal of Environmental Research and Public Health 2016;13(4):431 (24 pp.). |
R834514 (Final) R835738 (2016) R835738 (2017) R835738C005 (2015) R835738C005 (2016) R835738C005 (2017) |
Exit Exit Exit |
|
|
Smith MN, Workman T, McDonald KM, Vredevoogd MA, Vigoren EM, Griffith WC, Thompson B, Coronado GD, Barr D, Faustman EM. Seasonal and occupational trends of five organophosphate pesticides in house dust. Journal of Exposure Science and Environmental Epidemiology 2017;27(4):372-378. |
R834514 (Final) |
Exit |
|
|
Smith M, Hubal E, Faustman E. A Case study on the utility of predictive toxicology tools in alternatives assessments for hazardous chemicals in children's consumer products. JOURNAL OF EXPOSURE SCIENCE AND ENVIRONMENTAL EPIDEMIOLOGY 2020;30(1):160-170. |
R834514 (Final) |
Exit |
|
|
Souza-Nogueira A, Camargo AE, Remondi FA, Paoliello MM, Richter RJ, Furlong CE, Barbosa DS, Maes M, Moreira EG. Paraoxonase 1 (PON1) Q192R genotypes and their interaction with smoking strongly increase atherogenicity and the Framingham risk score. Archives of Endocrinology and Metabolism 2016;60(5):426-435. |
R834514 (Final) |
Exit Exit |
|
|
Sridharan G, Wamalwa D, John-Stewart G, Tapia K, Langat A, Moraa Okinyi H, Adhiambo J, Chebet D, Maleche-Obimbo E, Karr CJ, Benki-Nugent S. High viremia and wasting before antiretroviral therapy are associated with pneumonia in early-treated HIV-infected Kenyan infants. Journal of the Pediatric Infectious Diseases Society 2017;6(3):245-252. |
R834514 (Final) |
Exit Exit |
|
|
Stanaway IB, Wallace JC, Shojaie A, Griffith WC, Hong S, Wilder CS, Green FH, Tsai J, Knight M, Workman T, Vigoren EM, McLean JS, Thompson B, Faustman EM. Human oral buccal microbiomes are associated with farmworker status and azinphos-methyl agricultural pesticide exposure. Applied and Environmental Microbiology 2017;83(2):e02149-16. |
R834514 (Final) |
Exit Exit Exit |
|
|
Stanaway I, Wallace J, Hong S, WIlder C, Green F, Tsai J, Knight M, Workman T, Vigoren E, Smith M, Griffith W, Thompson B, Shojaie A, Faustman E. Alteration of oral microbiome composition in children living with pesticide-exposed farm workers. INTERNATIONAL JOURNAL OF HYGIENE AND ENVIRONMENTAL HEALTH 2023;248(114090). |
R834514 (Final) R832733 (Final) |
Exit |
|
|
Strong LL, Thompson B, Koepsell TD, Meischke H, Coronado GD. Reducing the take-home pathway of pesticide exposure:behavioral outcomes from the Para Niños Saludables study. Journal of Occupational and Environmental Medicine 2009;51(8):922-933. |
R834514 (Final) R834514C001 (Final) R831709 (2007) |
Exit |
|
|
Strong LL, Starks HE, Meischke H, Thompson B. Perspectives of mothers in farmworker households on reducing the take-home pathway of pesticide exposure. Health Education & Behavior 2009;36(5):915-929. |
R834514 (Final) R834514C001 (Final) |
Exit Exit Exit |
|
|
Suzuki SM, Stevens RC, Richter RJ, Cole TB, Park S, Otto TC, Cerasoli DM, Lenz DE, Furlong CE. Engineering human PON1 in an E. coli expression system. Advances in Experimental Medicine and Biology 2010;660:37-45. |
R834514 (2011) R834514 (2012) R834514 (2013) R831709 (2007) |
Exit |
|
|
Thompson B, Griffith WC, Barr DB, Coronado GD, Vigoren EM, Faustman EM. Variability in the take-home pathway: farmworkers and non-farmworkers and their children. Journal of Exposure Science & Environmental Epidemiology 2014;24(5):522-531. |
R834514 (Final) R834514C001 (Final) |
Exit Exit |
|
|
Ticozzi N, LeClerc AL, Keagle PJ, Glass JD, Wills AM, van Blitterswijk M, Bosco DA, Rodriguez-Levva I, Gellera C, Ratti A, Taroni F, McKenna-Yasek D, Sapp PC, Silani V, Furlong CE, Brown Jr. RH, Landers JE. Paraoxonase gene mutations in amyotrophic lateral sclerosis. Annals of Neurology 2010;68(1):102-107. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Wegner SH, Yu X, Pacheco Shubin S, Griffith WC, Faustman EM. Stage-specific signaling pathways during murine testis development and spermatogenesis: a pathway-based analysis to quantify developmental dynamics. Reproductive Toxicology 2015;51:31-39. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) R835738 (2016) R835738 (2017) R835738C004 (2015) R835738C004 (2017) |
Exit Exit Exit |
|
|
Wegner S, Hong S, Yu X, Faustman EM. Preparation of rodent testis co-cultures. Current Protocols in Toxicology 2013;Chapter 16:Unit 16.10. |
R834514 (2013) |
Exit |
|
|
Wegner S, Yu X, Kim HY, Harris S, Griffith WC, Hong S, Faustman EM. Effect of dipentyl phthalate in 3-dimensional in vitro testis co-culture is attenuated by cyclooxygenase-2 inhibition. Journal of Toxicology and Environmental Health Sciences 2014;6(8):161-169. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit |
|
|
Wegner S, Park J, Workman T, Workman T, Hermsan S, Wallace J, Stanaway I, Kim H, Griffith W, Hong S, Faustman E. Anchoring a dynamic in vitro model of human neuronal differentiation to key processes of early brain development in vivo. Reproductive Toxicology 2020;91:116-130. |
R834514 (Final) R835738 (2018) |
Exit Exit |
|
|
Wegner S, Workman T, Park J, Harris S, Wallace J, Stanaway I, Hong S, Hansen B, Griffith W, Faustman E. A dynamic in vitro developing testis model reflects structures and functions of testicular development in vivo. REPRODUCTIVE TOXICOLOGY 2023;118(108362) |
R834514 (Final) R835738 (Final) |
Exit |
|
|
Weiner MW, Meyerhoff DJ, Neylan TC, Hlavin J, Ramage ER, McCoy D, Studholme C, Cardenas V, Marmar C, Truran D, Chu PW, Kornak J, Furlong CE, McCarthy C. The relationship between Gulf War war illness, brain N-acetylaspartate, and post-traumatic stress disorder. Military Medicine 2011;176(8):896-902. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit |
|
|
Weldon BA, Shubin SP, Smith MN, Workman T, Artemenko A, Griffith WC, Thompson B, Faustman EM. Urinary microRNAs as potential biomarkers of pesticide exposure. Toxicology and Applied Pharmacology 2016;312:19-25. |
R834514 (Final) R834514C001 (Final) |
Exit Exit Exit |
|
|
Whitely Binder LC, Barcelos JK, Booth DB, Darzen M, Elsner MM, Fenske R, Graham TF, Hamlet AF, Hodges-Howell J, Jackson JE, Karr C, Keys PW, Littell JS, Mantua N, Marlow J, McKenzie D, Robinson-Dorn M, Rosenberg EA, Stockle CO, Vano JA. Preparing for climate change in Washington State. Climatic Change 2010;102(1-2):351-376. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C002 (Final) |
Exit Exit |
|
|
Woodruff TJ, Parker JD, Darrow LA, Slama R, Bell ML, Choi H, Glinianaia S, Hoggatt KJ, Karr CJ, Lobdell DT, Wilhelm M. Methodological issues in studies of air pollution and reproductive health. Environmental Research 2009;109(3):311-320. |
R834514 (2012) R834514 (2013) R834514 (Final) R833863 (2009) |
Exit Exit Exit |
|
|
Yu X, Robinson JF, Sidhu JS, Hong S, Faustman EM. A system-based comparison of gene expression reveals alterations in oxidative stress, disruption of ubiquitin-proteasome system and altered cell cycle regulation after exposure to cadmium and ethylmercury in mouse embryonic fibroblast. Toxicological Sciences 2010;114(2):356-377. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) R834514C003 (Final) R831709 (2007) |
Exit Exit |
|
|
Yu X, Sidhu JS, Hong S, Robinson JF, Ponce RA, Faustman EM. Cadmium induced p53-dependent activation of stress signaling, accumulation of ubiquitinated proteins, and apoptosis in mouse embryonic fibroblast cells. Toxicological Sciences 2011;120(2):403-412. |
R834514 (2011) R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Zachek CM, Karr CJ, Daniell W, Sweeney C, Miller MD. A network of Pediatric Environmental Health Specialty Units (PEHSUs): filling a critical gap in the health care system. Medycyna Środowiskowa--Environmental Medicine 2012;15(3):9-20. |
R834514 (2012) R834514 (2013) R834514 (Final) |
Exit Exit |
|
|
Zhou C, Chen J, Zhang X, Costa LG, Guizzetti M. Prenatal ethanol exposure up-regulates the cholesterol transporters ATP-binding cassette A1 and G1 and reduces cholesterol levels in the developing rat brain. Alcohol and Alcoholism 2014;49(6):626-634. |
R834514 (2015) R834514 (Final) R834514C003 (2015) R834514C003 (Final) |
Exit Exit Exit |
|
|
Marsillach, J., E.J. Hsieh, R.J. Richter, M.J. Maccoss, and C.E. Furlong, Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chem Biol Interact, 2012. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Crosslin, DR, Auer, PL, Suzuki, SM, Marsillach, J, Burt, AA, Gordon, AS, Meschia, JF, Nalls, MA, Worrall, BB, Longstreth, WT, Jr., Gottesman, RF, Furlong, CE, Peters, U, Rich, SS, Nickerson, DA and Jarvik, GP. 2014. Rare coding variation in paraoxonase-1 is associated with ischemic stroke in the NHLBI Exome Sequencing Project. J Lipid Res. 55(6):1173-1178. 4031948. |
R834514C004 (Final) |
not available |
|
|
Guizzetti, M., N.H. Moore, G. Giordano, K.L. VanDeMark, and L.G. Costa, Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia, 2010. 58(12):p. 1395-406. PMCID2925144. |
R834514C004 (Final) |
not available |
|
|
Richter RJ, Jarvik GP, Furlong CE. 2010. Paraoxonase 1 (PON1) Status as a Risk Factor for Disease or Exposure. Adv Exp Med Biol 660:29-35. PMID 20221868. DOI 10.1007/978-1-60761-350-3_. NIHMSID #193805. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Burt, AA, Ranchalis, JE, Richter, RJ, Marshall, JK, Nakayama, KS, Jarvik, ER, Eintracht, JF, Rosenthal, EA, Furlong, CE and Jarvik, GP. 2012. Dietary cholesterol increases paraoxonase 1 enzyme activity. J Lipid Res. 53(11):2450-8. PMCID:PMC3466014. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Burt, A, Ranchalis, J, Richter, RJ, Marshall, E, Rosenthal, E, Furlong, C and Jarvik, GP. 2012. Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. J Lipids 2012:476316. PMCID:PMC3364586. |
R834514C004 (Final) |
not available |
|
|
Costa, LG, Giordano, G, Cole, TB, Marsillach, J and Furlong, CE. 2013. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology. 307(10):115-122. PMCID:PMC3516631. |
R834514C004 (Final) |
not available |
|
|
Marsillach, J, Hsieh, EJ, Richter, RJ, MacCoss, MJ, Furlong, CE. 2013. Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures. Chem Biol Interact 203(1):85-90. |
R834514C004 (Final) |
not available |
|
|
Kim, DS, Burt, AA, Crosslin, DR, Robertson, PD, Ranchalis, JE, Boyko, EJ, Nickerson, DA, Furlong, CE and Jarvik, GP. 2013. Novel common and rare genetic determinants of paraoxonase activity:FTO, SERPINA12, and ITGAL. J Lipid Res. 54(2):552-60. PMCID:PMC3588879. |
R834514C004 (Final) |
not available |
|
|
Costa, LG, de Laat, R, Dao, K, Pellacani, C, Cole, TB and Furlong, CE. 2013. Paraoxonase-2 (PON2) in brain and its potential role in neuroprotection. Neurotoxicology. 3942372. |
R834514C004 (Final) |
not available |
|
|
Costa, LG, Tait, L, de Laat, R, Dao, K, Giordano, G, Pellacani, C, Cole, TB and Furlong, CE. 2013. Modulation of paraoxonase 2 (PON2) in mouse brain by the polyphenol quercetin:a mechanism of neuroprotection? Neurochem Res. 38(9):1809-18. 3735620. |
R834514C004 (Final) |
not available |
|
|
Cole, TB, Li, WF, Co, AL, Hay, AM, MacDonald, JW, Bammler, TK, Farin, FM, Costa, LG and Furlong, CE. 2014. Repeated Gestational Exposure of Mice to Chlorpyrifos Oxon is Associated with Paraoxonase 1 (PON1)-Modulated Effects in Maternal and Fetal Tissues. Toxicol Sci. |
R834514C004 (Final) |
not available |
|
|
Garrick JM, Dao K, de Laat R, Elsworth J, Cole TB, Marsillach J, Furlong CE, Costa LG. 2016. Developmental expression of paraoxonase 2. Chem Biol Interact. pii:S0009-2797(16)30116-8. doi:10.1016/j.cbi.2016.04.001. PMID:27062895. |
R834514C004 (Final) |
not available |
|
|
Furlong CE, Marsillach J, Jarvik GP, Costa LG. 2016. Paraoxonases-1,-2 and-3:What are their functions? Chem Biol Interact. doi:10.1016/j.cbi.2016.05.036. PMID:27238723. |
R834514C004 (Final) R834513C003 (2016) |
not available |
|
|
Marsillach J, Costa LG, Furlong CE. 2016. Paraoxonase-1 and Early-Life Environmental Exposures. Ann Glob Health. 82(1):100-10. PMID:27325068; PMCID:PMC4916371. |
R834514C004 (Final) |
not available |
|
|
Cole, T.B., J.C. Fisher, T.M. Burbacher, L.G. Costa, and C.E. Furlong, Neurobehavioral assessment of mice following repeated postnatal exposure to chlorpyrifos-oxon. Neurotoxicol Teratol, 2012. 34(3):p. 311-322. |
R834514C004 (Final) R834798C003 (Final) |
not available |
|
|
Burton C, Marsillach J, Zhang YS, Zhao W, Zheng X. Bioanalysis. 2015;7(13):1667-73. doi:10.4155/bio.15.90. Epub 2015 Jul 7** Judit Marsillach selected as a finalist for the Young Investigator Award to identify and reward promising early career researchers in the community. |
R834514C004 (Final) |
not available |
|
|
Furlong, C.E., R.J. Richter, L.G. Costa, and G.P. Jarvik, Paraoxonase 1 (PON1) Status in Risk Assessment for Organophosphate Exposure and Pharmacokinetics, in Chapter 9:Parameters for Pesticide QSAR and PBPK/PD Models for Human Risk Assessment. 2012, American Chemical Society:Available at:http://pubs.acs.org/doi/abs/10.1021/bk-2012-1099.ch009. p. 133-147. |
R834514C004 (Final) |
not available |
|
|
Costa, L.G., G. Giordano, and C.E. Furlong, Pharmacological and dietary modulators of paraxonase 1 (PON1) activity and expression:the hunt goes on. Biochem Pharmacol, 2011. 81(3):p. 337-344. PMCID3077125. |
R834514C004 (Final) |
not available |
|
|
Kim, D.S., A.A. Burt, J.E. Ranchalis, R.J. Richter, J.K. Marshall, K.S. Nakayama, E.R. Jarvik, J.F. Eintracht, E.A. Rosenthal, C.E. Furlong, and G.P. Jarvik, Dietary cholesterol increases paraoxonase 1 enzyme activity. J Lipid Res, 2012. 53(11):p. 2450-8. |
R834514C004 (Final) |
not available |
|
|
Kim, D.S., A. Burt, J. Ranchalis, R.J. Richter, E. Marshall, E. Rosenthal, C. Furlong, and G.P. Jarvik, Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. J Lipids, 2012. 2012(http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3364586/pdf/JL2012-476316.pdf). |
R834514C004 (Final) |
not available |
Supplemental Keywords:
RFA, Health, Scientific Discipline, INTERNATIONAL COOPERATION, ENVIRONMENTAL MANAGEMENT, Biochemistry, Children's Health, Environmental Policy, Biology, Risk Assessment, pesticide exposure, age-related differences, pesticides, children's vulnerablity, biological markers, agricultural communityProgress and Final Reports:
Original Abstract Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R834514C001 Community-Based Participatory Research
R834514C002 Pesticide Exposure Pathways
R834514C003 Molecular Mechanisms
R834514C004 Genetic Susceptibility
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2015 Progress Report
- 2014
- 2013 Progress Report
- 2012 Progress Report
- 2011 Progress Report
- 2010
- Original Abstract
178 journal articles for this center