Grantee Research Project Results
2009 Progress Report: Project 2: The Role of Oxidative Stress in PM-induced Adverse Health Effects
EPA Grant Number: R832413C002Subproject: this is subproject number 002 , established and managed by the Center Director under grant R832413
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Great Lakes Air Center for Integrative Environmental Research
Center Director: Harkema, Jack
Title: Project 2: The Role of Oxidative Stress in PM-induced Adverse Health Effects
Investigators: Nel, Andre E. , Kleinman, Michael T. , Lusis, Aldons , Harkema, Jack
Institution: University of California - Los Angeles , Michigan State University , University of California - Irvine
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
Project Period Covered by this Report: August 1, 2008 through July 31,2009
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
Progress Summary:
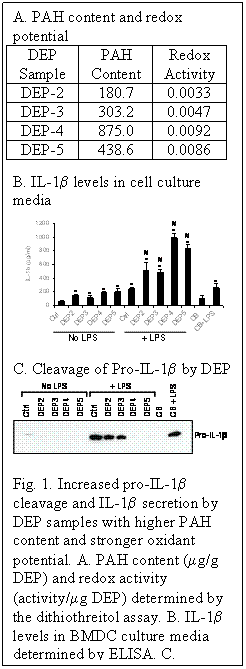 In the past few years, a new concept termed “inflammasome” has been introduced as a molecular sensor to explain how the innate immune system functions. The NOD-like receptors (NLR), a family of intracellular sensors of microbial motifs and ‘danger signals’, have been considered as being crucial components of the innate immune responses and inflammation. The NALP3 inflammasome is a caspase-1-activating multiprotein complex that processes proinflammatory cytokines. This inflammasome detects a number of danger signals such as bacterial muramyldipeptide, monosodium urate crystals and reactive oxygen species (ROS), which leads to assembly of it subunits and association with pro-caspase-1. Through the ability to activate caspase-1 and subsequent release of IL-1b from it its pro-peptide form, the NALP3 inflammasome plays an important role in the immunomodulatory effects of danger signals. In addition, it has been demonstrated that alum, a classical adjuvant for murine asthma model, stimulates inflammatory dendritic cells (DC) through the activation of NALP3 inflammasome. Based on these published reports, we have begun to investigate the role of NALP3 inflammasome in the adjuvant effect of ambient PM with a focus on DC. Using four out of seven DEP samples that we recently obtained from U.S. Environmental Protection Agency, we have found that the ability of these DEP collections to increase IL-1b secretion from bone marrow-derived DC (BMDC) is linked to their oxidant potential (Fig. 1A and 1B). Moreover, the ability of these DEP samples to cleave lipopolysaccharide (LPS)-induced pro-IL-1b was also associated to their PAH content and oxidant potential (Fig. 1A and 1C). Taken together, our preliminary data suggest that pro-oxidative PM may exert its adjuvant effect through the activation of NALP3 inflammasome in DC.
In the past few years, a new concept termed “inflammasome” has been introduced as a molecular sensor to explain how the innate immune system functions. The NOD-like receptors (NLR), a family of intracellular sensors of microbial motifs and ‘danger signals’, have been considered as being crucial components of the innate immune responses and inflammation. The NALP3 inflammasome is a caspase-1-activating multiprotein complex that processes proinflammatory cytokines. This inflammasome detects a number of danger signals such as bacterial muramyldipeptide, monosodium urate crystals and reactive oxygen species (ROS), which leads to assembly of it subunits and association with pro-caspase-1. Through the ability to activate caspase-1 and subsequent release of IL-1b from it its pro-peptide form, the NALP3 inflammasome plays an important role in the immunomodulatory effects of danger signals. In addition, it has been demonstrated that alum, a classical adjuvant for murine asthma model, stimulates inflammatory dendritic cells (DC) through the activation of NALP3 inflammasome. Based on these published reports, we have begun to investigate the role of NALP3 inflammasome in the adjuvant effect of ambient PM with a focus on DC. Using four out of seven DEP samples that we recently obtained from U.S. Environmental Protection Agency, we have found that the ability of these DEP collections to increase IL-1b secretion from bone marrow-derived DC (BMDC) is linked to their oxidant potential (Fig. 1A and 1B). Moreover, the ability of these DEP samples to cleave lipopolysaccharide (LPS)-induced pro-IL-1b was also associated to their PAH content and oxidant potential (Fig. 1A and 1C). Taken together, our preliminary data suggest that pro-oxidative PM may exert its adjuvant effect through the activation of NALP3 inflammasome in DC.
Future Activities:
1. We will continue to work on the OVA adjuvancy model including performing studies that will investigate the role of oxidative stress at the level of antigen presenting cells as outlined in our studies using dendritic cells and diesel exhaust particle extracts in year two.We will determine whether the adjuvant effect of ambient PM and DEP is mediated though the activation of inflammasome in DC. We will also assess whether PM-induced oxidative stress could act as a “danger signal” to DC. We will continue to validate our intranasal sensitization mouse model using seven different DEP samples collected by EPA. Characterization studies have shown that these DEP samples have different chemical composition and oxidant potential (Fig. 1A). They also differ in their ability to induce cellular oxidative stress and pro-inflammatory cytokine production, i.e. IL-8 and IL-1b. We will determine whether our mouse model could discern the differences among these DEP samples based on their differences in their chemical composition and oxidant potential. In collaboration with Dr. Sioutas and Dr. Harkema, we will also use our in vivo model to compare the ambient PM samples collected in different cities in the country, i.e. Los Angeles and Detroit, to determine how differences in ambient PM chemical composition affect asthma disease severity.
References:
Journal Articles on this Report : 8 Displayed | Download in RIS Format
| Other subproject views: | All 34 publications | 23 publications in selected types | All 23 journal articles |
|---|---|---|---|
| Other center views: | All 241 publications | 157 publications in selected types | All 157 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circulation Research 2008;102(5):589-596. |
R832413 (2008) R832413 (2009) R832413 (2010) R832413 (Final) R832413C001 (2008) R832413C001 (Final) R832413C002 (2007) R832413C002 (2008) R832413C002 (2009) R832413C002 (Final) R832413C003 (Final) |
Exit Exit Exit |
|
|
Chatila TA, Li N, Garcia-Lloret M, Kim H-J, Nel AE. T-cell effector pathways in allergic diseases:transcriptional mechanisms and therapeutic targets. Journal of Allergy and Clinical Immunology 2008;121(4):812-823. |
R832413 (2007) R832413 (2008) R832413 (2010) R832413 (Final) R832413C002 (2007) R832413C002 (2008) R832413C002 (2009) |
Exit Exit Exit |
|
|
Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radical Biology and Medicine 2008;44(9):1689-1699. |
R832413 (2007) R832413 (2008) R832413 (2010) R832413 (Final) R832413C002 (2007) R832413C002 (2008) R832413C002 (2009) |
Exit Exit Exit |
|
|
Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, Harkema JR, Nel AE. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environmental Health Perspectives 2009;117(7):1116-1123. |
R832413 (2009) R832413 (2010) R832413 (Final) R832413C001 (2009) R832413C001 (Final) R832413C002 (2009) R832413C002 (Final) |
|
|
|
Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008;2(5):889-896. |
R832413 (2010) R832413 (Final) R832413C002 (2009) |
Exit Exit Exit |
|
|
Xia T, Kovochich M, Liong M, Zink JI, Nel AE. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008;2(1):85-96. |
R832413 (2010) R832413 (Final) R832413C002 (2009) |
Exit Exit Exit |
|
|
Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008;2(10):2121-2134. |
R832413 (2010) R832413 (Final) R832413C002 (2009) |
Exit Exit Exit |
|
|
Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annual Review of Public Health 2009;30:137-150. |
R832413 (2008) R832413 (2010) R832413 (Final) R832413C002 (2009) |
Exit Exit |
Supplemental Keywords:
Asthma, atherosclerosis, oxidative stress, ambient PM, health effects, sensitive populations, human health, animal, PAH, gene co-expression network analysis, synergy, dendritic cell, adjuvant, Health, RFA, Scientific Discipline, Air, Health Risk Assessment, Risk Assessments, particulate matter, Biochemistry, Toxicology, Ecology and Ecosystems, human exposure, airborne particulate matter, animal model, cardiovascular vulnerability, oxidative stress, particulates, vascular dysfunction, atmospheric particulate matter, air pollution, cardiovascular disease, human health risk, human health effects, airway diseaseRelevant Websites:
Progress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832413 Great Lakes Air Center for Integrative Environmental Research Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832413C001 Contribution of Primary and Secondary PM Sources to Exposure & Evaluation of Their Relative Toxicity
R832413C002 Project 2: The Role of Oxidative Stress in PM-induced Adverse Health Effects
R832413C003 The Chemical Properties of PM and their Toxicological Implications
R832413C004 Oxidative Stress Responses to PM Exposure in Elderly Individuals With Coronary Heart Disease
R832413C005 Ultrafine Particles on and Near Freeways
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2011
- 2010 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
23 journal articles for this subproject
Main Center: R832413
241 publications for this center
157 journal articles for this center
