Grantee Research Project Results
2010 Progress Report: Project 2: The Role of Oxidative Stress in PM-induced Adverse Health Effects
EPA Grant Number: R832413C002Subproject: this is subproject number 002 , established and managed by the Center Director under grant R832413
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Great Lakes Air Center for Integrative Environmental Research
Center Director: Harkema, Jack
Title: Project 2: The Role of Oxidative Stress in PM-induced Adverse Health Effects
Investigators: Nel, Andre E. , Kleinman, Michael T. , Harkema, Jack , Lusis, Aldons
Current Investigators: Nel, Andre E. , Kleinman, Michael T. , Lusis, Aldons , Harkema, Jack
Institution: University of California - Los Angeles , Michigan State University , University of California - Irvine
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
Project Period Covered by this Report: August 1, 2009 through July 31,2010
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
The primary objective is to elucidate the mechanism(s) of particulate matter (PM)-induced asthma and atherosclerosis exacerbation. Mechanisms are investigated by performing in vivo animal studies in a mobile trailer suitable for exposure to ambient PM as well as executing in vitro studies of tissue culture cells. Our goal is to build a predictive toxicological paradigm in which the in vitro mechanisms of injury also are applicable to disease pathogenesis in vivo.
Progress Summary:
1. Progress on studies looking at the boosting effect of “real-life” ambient particles on the secondary immune response.
After we published our work on the adjuvant effect of ambient ultrafine particles (UFP) in the primary immune response and how that leads to allergic sensitization (Li, et al., Environ Health Perspect, 2009), we continued our effort to answer the question whether inhalation of ambient UFP could boost the secondary immune response in previously sensitized animals during an allergen response.
In collaboration with Dr. Sioutas, we completed our “real-life” inhalation exposure studies in our mobile animal research laboratory located near the 110 freeway in downtown Los Angeles (Li, et al., Am J Physiol Lung Cell Mol Physiol, 2010). Mice were sensitized by two intranasal administrations of OVA (10 μg) together with UFP (0.5 μg). After a 2-week break, animals were given inhalation exposures to either filtered air or concentrated ambient UFP for 4 hrs per day for 5 days. OVA aerosol (1%) challenge was given on the last two days of inhalation exposure. As few as five inhalation exposures to “real-life” UFP were sufficient to boost the secondary immune response during OVA challenge compared with the adjuvant effect of UFP on the primary immune response. The UFP-enhanced secondary immune response was characterized by a profound eosinophilic airway inflammation, increased systemic OVA-IgG1 and OVA-IgE production and Th2 and IL-17 cytokine gene expression profiles in the lung. Real-time PCR analysis confirmed that UFP inhalation significantly increased the expression of eotaxin, IL-5, IL-13, TNFα, KC, IL-10, and Muc5ac genes in the lungs of prior sensitized animals. In addition, UFP inhalation also enhanced IL-17a gene expression in the lung, which was accompanied by increased BAL neutrophil counts. Lung morphometry showed that UFP targeted the centriacinar region of the distal lung during the secondary immune response, which stands in contrast to the adjuvant effect of UFP on the primary immune response that elicits increased Ym1 protein expression, a particulate matter (PM)-induced oxidative stress marker previously identified by proteome analysis, in the same centriacinar region suggests that the pro-inflammatory effects of UFP during the secondary immune response involve the generation of local oxidative stress (Li, et al., Am J Physiol Lung Cell Mol Physiol, 2010). Taken together, the significance of this work is that our results show for the first time that pro-oxidative UFP inhalation exposure is quite effective in boosting the secondary immune response after only a limited number of exposures. This leads to the exacerbation of allergic airway inflammation characterized by Th2 and Th17 cytokine profiles in already-sensitized animals. Inhaled UFPs target the distal lung including the alveolar duct and alveolar parenchyma where the generation of oxidative stress may play a role in enhancing the secondary immune response. Our study also is important from the perspective of asthma prevention and exacerbation by air pollution particles. Epidemiological studies indicate a close link between surges in ambient PM levels and asthma flares. While a host of atmospheric conditions and pollutant sources may contribute to these exacerbations, it is important to consider the role of proximity to freeways in determining UFP concentrations.
2. Monitoring the adjuvant effect of ambient UFP by proteomics of broncholaveolar lavage fluid (BALF).
In collaboration with Dr. Joseph Loo from the Keck Proteomics facility at UCLA, we have completed proteomic profiling to identify new proteins that may be useful biomarkers for allergic inflammation related to air pollutants. We used 2D-PAGE (polyacrylamide gel electrophoresis) coupled with mass spectrometry to identify oxidative stress markers in BALF and lung tissue in our highly sensitive murine intranasal sensitization model (Li, et al., Environ Health Perspect, 2009) and to assess whether the adjuvant effect of ambient PM could lead to an altered proteome profile in BALF. We hypothesized that intranasal exposure to an extremely low dose of ambient pro-oxidative UFP, together with a low dose of allergen OVA is sufficient to change the proteome profile in the BALF and this alteration may be used to develop biomarkers to screen for the adjuvant effect of pro-oxidative PM. We showed that intranasal exposure to a precise amount of ambient PM was able to promote a Th2 immune response characterized by enhanced allergic airway inflammation and that the adjuvant effect of UFP was closely correlated to a significant change in the proteome profile in BALF (Kang, et al., Proteomics, 2010).
BALF proteins from control and sensitized mice were resolved by two-dimensional gel electrophoresis and identified by mass spectrometry. A total of 30 protein spots from 16 proteins were significantly changed in OVA sensitized mice and even more significantly changed in mice exposed to an OVA plus UFP combination. The 7 proteins showing the highest protein expression level changes were confirmed by western blotting and RT- PCR. Polymeric immunoglobulin receptor (PIGR), complement C3, neutrophil gelatinase-associated lipocalin (NGAL), chitinase 3-like protein 3 (Ym1), chitinase 3-like protein 4 (Ym2), and acidic mammalian chitinase (AMcase) demonstrated significantly enhanced up-regulation by the UFP that exhibits a high polycyclic aromatic hydrocarbon (PAH) content and high oxidant potential. The significance of this study is that these proteins may be the important specific response elements for PM through its ability to generate reactive oxygen species in the mucosal immune system. These proteins may be involved in allergen sensitization and asthma pathogenesis. This work has been published in Proteomics (Kang, et al., Proteomics, 2010).
In addition, we have conducted proteome analysis of the BALF and lung samples obtained from our “real-life” inhalation exposure study (Li, et al., Am J Physiol Lung Cell Mol Physiol, 2010) in order to identify the potential biomarkers that are associated with the boosting effect of ambient UFP on the secondary immune response. This analysis is in progress.
3. Progress on assessing the role of dendritic cells (DC) in executing the adjuvant effects of PM using adoptive transfer of PM-exposed dendritic cells for allergic sensitization.
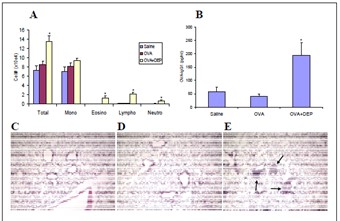
Dendritic cells play a central role in regulating immune response including allergic airway inflammation such as asthma. Last year, we reported that the ability of different diesel exhaust particle (DEP) samples to stimulate IL-1β secretion from bone marrow-derived DC (BMDC) and to cleave LPS-induced pro-IL-1β is associated with PAH content and oxidant potential. Recently, we also made progress in studying the role of DC in executing the adjuvant effects of DEP. BMDCs from Balb/C mice were pulsed with saline, OVA (50 μg/ml) or OVA (50 μg/ml) plus DEP (10 μg/ml) for 16 hrs before the intra-tracheal adoptive transfer of these cells into the recipient Balb/C mice for allergic sensitization. After a 2-week break, the recipient animals received two daily OVA (1%) aerosol challenges and necropsy was conducted 48 hrs later. Our results have shown that DEP was capable of enhancing BMDC function which, upon adoptive transfer, could result in an enhanced allergic sensitization to OVA and subsequent airway inflammation in the recipient animals with enhanced eosinophilic inflammation in the lung and increased OVA-IgG1 level in the plasma (Figure 1). We currently are conducting studies to explore the role of inflammasome and oxidative stress in mediating this effect of DEP on BMDC. This will be completed in the no-cost extension period.
Figure 1. Enhanced allergic airway inflammaation in Balb/C
mice sensitized by adobpitive transfer of OVA/DEP-pulsed
BMDC. A: Increased BAL eosinophil in mice sensitized by
OBA/DEP-treated BMDC. B: Increased plasma OVA-IgG1
level in the animals sensitized by OVA/DEP-pulsed BMDC.
C-E: Histological analysis showing that OVA/DEP-pulsed
BMDC (E) induced allergic airway inflammation in vivo
compared with the saline (C) and OVA (D) pulsed BMDC.
*p<0.05 compared with saline and OVA. Arrows indicate
inflammation in the lung.
Future Activities:
Proposed no-cost extension activities to close out our research
We have been granted an 18-month no-cost extension to complete our studies. During this period, we will complete the following remaining aspects of our study:
1. Use of normal and genetically susceptible mice to study the role of oxidative stress in PM-induced exacerbation of asthma. We have successfully backcrossed Nrf2 knockout (Nrf2KO) animals onto a Balb/c background, which allows us to study the role of oxidative stress in the adjuvant effect of PM due to the ability of this mouse strain to develop stronger Th2 immune responses during the respiratory sensitization to OVA. The remaining work that needs to be accomplished in this strain will use low grade OVA sensitization to study the effects of fine and UFPs on the exacerbation of allergic airway inflammation, oxidative stress, IgE production, mucus hyper-secretion, and airway hyperreactivity (AHR) in wild-type (wt) and Nrf2KO animals. We propose that the weakened antioxidant defense due to the Nrf2 deficiency will augment OVA sensitization and Th2 differentiation in the Balb/c background. We also will use BMDC from wt and Nrf2KO mice to conduct adoptive transfer experiments.
2. Study the role of dendritic cells in executing the adjuvant effects of PM due to the putative ability of pro-oxidative components to induce the assembly of an inflammazone that dictates Th2 differentiation of the immune response. We will complete our studies to investigate the role of pro-oxidative PM components in the assembly of the NALP3 inflammasome in DC that may polarize Th2 differentiation of the immune response. This will help us to further refine our understanding of the adjuvant effect of particulate matter in the immune system. We will identify PM components that are responsible for inflammasome activation in DC and subsequent enhancement of allergic airway inflammation in vivo after sensitization by adoptive transfer. PM of different sizes, chemical composition as well as oxidant potential will be studied in BMDC. We will perform ELISA to analyze the IL-1β production by DC. Inflammasome activation will be confirmed by analyzing pro-IL-1β and pro-caspase-1 cleavage using immunoblotting. The correlation between PM-induced NALP3 inflammasome activation and the ability of the particles or their chemical components to induce adjuvant effect in the animal airway will be evaluated.
3. Proteomic profiling of BALF to identify the biomarkers associated with the adjuvant effect of ambient UFP on the secondary immune response. We will complete our proteomic analysis on the BALF and lung samples from our “real-life” inhalation exposure study (Li, et al., Am J Physiol Lung Cell Mol Physiol, 2010) in order to identify the potential biomarkers that are associated with the boosting effect of ambient UFP on the secondary immune response.
Journal Articles on this Report : 5 Displayed | Download in RIS Format
| Other subproject views: | All 34 publications | 23 publications in selected types | All 23 journal articles |
|---|---|---|---|
| Other center views: | All 241 publications | 157 publications in selected types | All 157 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Araujo JA, Nel AE. Particulate matter and atherosclerosis:role of particle size, composition and oxidative stress. Particle and Fibre Toxicology 2009;6:24. |
R832413 (Final) R832413C002 (2010) R832413C002 (Final) |
Exit Exit Exit |
|
|
George S, Pokhrel S, Xia T, Gilbert B, Ji Z, Schowalter M, Rosenauer A, Damoiseaux R, Bradley KA, Madler L, Nel AE. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 2010;4(1):15-29. |
R832413 (Final) R832413C002 (2010) |
Exit Exit Exit |
|
|
Kang X, Li N, Wang M, Boontheung P, Sioutas C, Harkema JR, Bramble LA, Nel AE, Loo JA. Adjuvant effects of ambient particulate matter monitored by proteomics of bronchoalveolar lavage fluid. Proteomics 2010;10(3):520-531. |
R832413 (Final) R832413C002 (2010) R832413C002 (Final) |
Exit |
|
|
Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, Ning Z, Kleinman MT, Sioutas C, Nel AE. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response:implication for traffic-related asthma flares. American Journal of Physiology 2010;299(3):L374-L383. |
R832413 (Final) R832413C001 (2010) R832413C001 (Final) R832413C002 (2010) R832413C002 (Final) |
Exit Exit Exit |
|
|
Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials 2009;8(7):543-557. |
R832413 (Final) R832413C002 (2010) |
Exit |
Supplemental Keywords:
Secondary immune response, ambient ultrafine particles, inhalation, distal lung, allergic inflammation, IL-17a, proteomics, inflammasome, dendritic cell, PM components, oxidative stress, Health, RFA, Scientific Discipline, Air, Health Risk Assessment, Risk Assessments, particulate matter, Biochemistry, Toxicology, Ecology and Ecosystems, human exposure, airborne particulate matter, animal model, cardiovascular vulnerability, oxidative stress, particulates, vascular dysfunction, atmospheric particulate matter, air pollution, cardiovascular disease, human health risk, human health effects, airway diseaseProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832413 Great Lakes Air Center for Integrative Environmental Research Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832413C001 Contribution of Primary and Secondary PM Sources to Exposure & Evaluation of Their Relative Toxicity
R832413C002 Project 2: The Role of Oxidative Stress in PM-induced Adverse Health Effects
R832413C003 The Chemical Properties of PM and their Toxicological Implications
R832413C004 Oxidative Stress Responses to PM Exposure in Elderly Individuals With Coronary Heart Disease
R832413C005 Ultrafine Particles on and Near Freeways
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2011
- 2009 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
23 journal articles for this subproject
Main Center: R832413
241 publications for this center
157 journal articles for this center