Grantee Research Project Results
Final Report: Project B: Exposure Project: Mn, DDT/E and PBDE Exposure to Farmworker Children
EPA Grant Number: R834513C002Subproject: this is subproject number 002 , established and managed by the Center Director under grant R834513
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Center for Research on Early Childhood Exposure and Development in Puerto Rico
Center Director: Alshawabkeh, Akram
Title: Project B: Exposure Project: Mn, DDT/E and PBDE Exposure to Farmworker Children
Investigators: Eskenazi, Brenda , Harley, Kim , Holland, Nina T. , Jerrett, Michael , Sjodin, Andreas , Arora, Manish , Smith, Donald , Eisen, Ellen , Molitor, John , Hubbard, Alan , Lustig, Robert
Institution: University of California - Berkeley
EPA Project Officer: Hahn, Intaek
Project Period: August 1, 2009 through July 31, 2014 (Extended to July 31, 2017)
RFA: Children's Environmental Health and Disease Prevention Research Centers (with NIEHS) (2009) RFA Text | Recipients Lists
Research Category: Children's Health , Human Health
Objective:
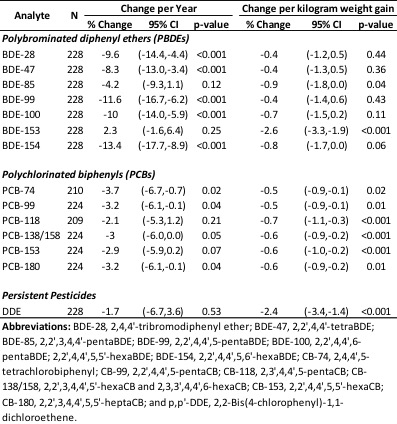
In Project B, we examined novel methods of assessing prenatal exposure to Mn, PBDE, and DDT/E compounds. For Mn, we developed methods to measure Mn in shed deciduous teeth and in hair collected when the children were 10.5 years of age. For PBDEs and DDT/E, we developed models to back-extrapolate prenatal exposure levels from 9-year measured levels and other determinants of exposure. We identified predictors of PBDE levels in 9 year old children and found that the geometric means for these compounds decreased significantly (between 8 and 13 percent per year) in the children from age seven to nine years (Table 2). We also showed that BDE-153, the PCBs and DDE all decreased significantly per kilogram weight gain in the child related to dilution from an increasing body mass with age.
| Project B Specific Aims: 1. To measure Mn, PBDEs, and DDT/E in shed deciduous teeth. To determine the best and most convenient matrix for assessing children’s exposure to OPs. 2. To determine the relationship between Mn, PBDE, and DDT/E levels in teeth with other relevant biological measures. 3. To quantify the contribution of agricultural pesticide use to Mn levels in house dust, teeth, hair and child blood. 4. To identify population correlates of PBDEs and DDT/E exposure in 9-year-old boys. |
Summary/Accomplishments (Outputs/Outcomes):
1. Mn in shed teeth: We successfully developed methods for measuring Mn in human teeth by laser ablation ICP-mass spectrometry (LA/ICP/MS), including analysis of 307 teeth collected from the CHAMACOS cohort. Rather thancalculate one estimate of cumulative exposure over the entire prenatal period, we were able to distinguish Mnexposure experienced in the 2nd trimester from that experienced in the 3rd trimester. We observed a significant association (rspearman =0.36, p=0.001, n=77) between Mn levels in the entire prenatally-formed dentine [as defined by 55Mn:43Ca area under the curve (AUC)] with floor dust Mn loading (µg Mn/m2 floor area) and a stronger relationship with Mn levels in dentine formed only in the 2nd trimester (rspearman =0.40; p=0.0005; n=72), likelybecause dust samples were collected in the 2nd trimester. We have also compared Mn levels in cord blood andmantle dentine. While there was no significant association of 55Mn:43Ca AUC of all prenatal sampling points withcord blood Mn (rspearman =-0.01; p=0.99), 55Mn:43Ca in the sampling point immediately adjacent to the neonatal line in each tooth showed a significant positive correlation with cord blood Mn (rspearman =0.70; p=0.003; n=16).These findings were published in Environmental Science & Technology (Arora et al. 2012) and the measurements were used in subsequent studies examining exposure and health outcomes (see below and Progress Report A).
2. PBDEs and DDT/E in shed teeth: As a pilot investigation, we assessed the feasibility of measuring persistent organic pollutants in deciduous teeth. Dr. Andreas Sjödin at the CDC in Atlanta attempted to measure PBDEs andDDT in anonymous deciduous teeth collected from dental patients demographically similar to the CHAMACOScohort. Using whole tooth digestion, neither PBDEs nor DDT were detected. Additional anonymous deciduous teethwere obtained from a local clinic and Dr. Arora extracted the dentine from these teeth to determine if more sensitiveanalyses could be employed to measure PBDEs and organochlorine (OC) compounds including DDT. Initial results were not promising. Specifically, low detection frequencies for the typically prevalent congeners PBDE-47 and -99 and high concentrations of PBDE-183 for all samples suggested possible contamination with PBDE-183. One challenge in the interpretation of xenobiotic measurements in dentine is the likelihood that dentine tissue, which is vasculated, may be in equilibrium with child exposures when the teeth are shed and may not reflect prenatal exposures. Some compounds, such as metals like manganese, are incorporated into the chemical structure of the dentine, but many organic compounds are not. Additional research is needed to broadly screen for chemicals in dentine strata and validate measurements against maternal and child biomonitoring.
3. Relationship between Mn levels in teeth with other relevant biological measures. For a subset of CHAMACOS participants, we conducted laboratory measurements of Mn in multiple biological samples, including202 whole blood samples (maternal blood, cord blood, child’s blood), 128 urine samples (maternal at 26-weekgestation and child at 24 months), and 62 teeth and examined the interrelationships of Mn levels in all matrices. Mn levels (Mn:Ca ratio) were higher in prenatal than postnatal dentine (geometric mean (GM) = 0.51 vs. 0.16, p<0.0001). Maternal blood Mn concentrations increased from 26 weeks gestation to delivery (GM = 14.6 to 20.7 µg/L, p = 0.001) and child blood Mn concentrations decreased from cord blood to 24-month blood samples (39.9 vs. 25.0 µg/L, p = 0.005). Mn levels in tooth dentine during the 3rd trimester were positively correlated with Mn concentrations in cord blood (rs = 0.31), while there was a negative correlation between prenatal Mn levels in enamel and concentrations in maternal blood at 26-weeks’ gestation (rs = -0.36). We did not find any significantdifferences in Mn urine concentrations over time and did not observe significant correlations between Mn levels inteeth and urine, suggesting that urinary concentrations are not a useful measure of environmental Mn exposure.We observed significantly higher (p < 0.05) levels of Mn in prenatal dentine, prenatal maternal blood, and 24-month urine from children if there was a farm worker living in the home during the corresponding time period compared to no farmworker living in the home. Prenatal Mn levels in dentine and cord blood were also correlated with Mn loading in prenatal house dust samples (rs = 0.27 and 0.29, respectively; p < 0.1). Tooth dentine and blood Mn concentrations had the strongest associations with potential sources of Mn exposure in the home. Our results were published in Environmental Science & Technology (Gunier et al. 2014).
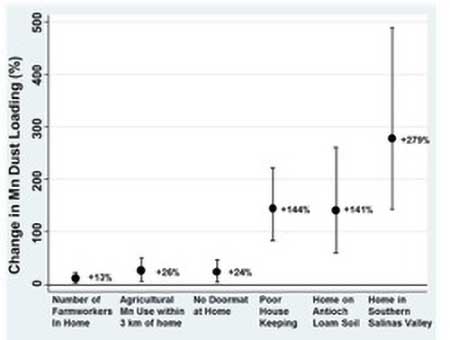
4. Mn in dust: We collected house dust samples from 378 CHAMACOS participant residences, with a second sample collected approximately nine months later from 90 of the residences. House dust samples were analyzed for Mn using inductively coupled plasma optical emission spectroscopy. Information from interviews, home inspections, and pesticide use reports was used to identify potential predictors of Mn dust concentrations and loadings. Mn was detectable in all dust samples. The median Mn concentration was 171 µg/g and median Mn loading was 1,910 µg/m2at first visit. In multivariable models, Mn dust concentrations and loadings increased with the number of farmworkers in the home and the amount of agricultural Mn fungicides applied within three kilometers of the residence during the month prior to dust sample collection. Dust concentrations of Mn and other metals (lead, cadmium and chromium) were higher in residences located in the southern Salinas Valley compared to those located in other areas of the Salinas Valley. Dust loadings of Mn and other metals were also higher in residences located on Antioch Loam soil than other soil types, and in homes with poor or average housekeeping practices (Figure B1). Agricultural use of Mn-containing fungicides was associated with Mn dust concentrations and loadings in nearby residences and farmworker homes. Housekeeping practices and soil type at residence were also important factors related to dust metal concentrations and loadings. These findings were published in Science of the Total Environment (Gunier et al., 2014).
|
|
| Table B1. Percent change of Mn in prenatala dentine and partial coefficient of determination (r2) for predictors in multivariable models for tooth Mn (n=206) and subset with tooth and dust Mn (n=130). | ||||||
| Children with Tooth Mn Levels | Children with Tooth and Dust Mn | |||||
| Predictor Variable | % Changeb (95% CI) | p-value | Partial r2 (%) | % Changeb (95% CI) | p-value | Partial r2 (%) |
| Maternal farmwork | 10.1 (0.1, 21) | 0.05 | 1.8 | 15.8 (1.9, 31.6) | 0.03 | 3.9 |
| (Prenatal yes vs. no) | ||||||
| Farm worker shoes in home | 8.1 (4.3, 12) | <0.001 | 8.4 | 6.4 (1.5, 11.4) | 0.01 | 5.2 |
| (Prenatal per worker) | ||||||
| Agricultural Fungicide Use Prenatal within 3 km | 4.9 (0.9, 9.1) | 0.02 | 2.8 | 3.4 (-1.6, 8.6) | 0.19 | 1.4 |
| (per IQRc = 809 kg ) | ||||||
| Soil Type | 15 (4.6, 27) | 0.004 | 4.0 | 21 (6.3, 37) | 0.004 | 6.4 |
| (Antioch Loam vs. other) | ||||||
| Mother smoked | -34 (-47, -18) | <0.001 | 6.7 | -40 (-55, -20) | 0.001 | 9.0 |
| (Prenatal yes vs. no) | ||||||
| Mn dust loading | - | - | 3.3 (0.3, 6.4) | 0.03 | 3.6 | |
| (per IQRc = 1465 µg/m2) | ||||||
| R2 for Model |
|
| 22% |
|
| 29% |
| aPrenatal = 2nd and 3rd trimesters. bPercent change = (exp(β)-1)*100; cIQR = interquartile range. | ||||||
5. Mn in teeth: We examined environmental and lifestyle factors associated with prenatal tooth dentine Mn concentrations for 207 CHAMACOS children. We found that storage of farmworkers’ shoes in the home, maternal farm work, agricultural use of Mn-containing fungicides within 3 km of the residence, residence built on Antioch Loam soil and Mn dust loading (µg/m2 of floor area) during pregnancy were associated with higher Mn levels in prenatal dentine (p<0.05). Maternal smoking during pregnancy was inversely related to Mn levels in prenatal dentine (p<0.01). Multivariable regression models explained 22 – 29% of the variability of Mn in prenatal dentine (Table B1). Our results suggest that Mn measured in prenatal dentine provides retrospective and time-specific levels of fetal exposure resulting from environmental and occupational sources (Gunier et al. 2013).
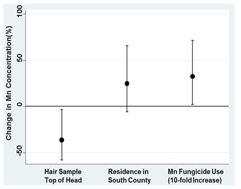
6. Mn in hair: In the absence of a validated methodology for the accurate determination of hair Mn without external contamination, we developed and validated a method to analyze Mn in hair which included thorough sample cleanup, showing in the process that prior published studies of hair Mn are likely erroneous (Eastman et al 2013). We havecollected hair samples from 455 children (145 boys and 310 girls). Due to costs associated with the extensive cleanup procedure necessary to analyze the hair properly, we were only able to analyze hair from a subsample of children. Dr. Smith at UCSC has analyzed samples from 135 participants. The Mn hair concentrations in our cohort (median=0.08 µg/g) were similar (median=0.07 µg/g) to the only other published study using the same cleaning methodology, which focused on children exposed to Mn through proximity to a ferroalloy plant (Eastman et al. 2013). In bivariate analyses, we found that Mn hair concentrations were related (p<0.2) to location of the hair sample (lower in samples from the top of the head), soil type at the child’s residence (higher in Antioch Loam), location of the residence in the Southern portion of Monterey County (higher in the South) and agricultural use of Mn fungicides within 3 km of the child’s residence during the 6 months prior to sample collection. Hair Mn concentrations were not related to demographic variables, the presence of agricultural workers in the home or the use of hair care products prior to sample collection. Location of hair sample (p=0.04), residence in Southern Monterey County (p=0.13) and agricultural use of Mn fungicides near the child’s residence (p=0.04) remained significant in multivariate models (Figure B2) and this model explained 12% of the variability in Mn hair concentrations. We did not observe an association between hair Mn concentrations and 10.5 year IQ or behavior in models adjusted for child’s exact age, maternal education, poverty status, language of assessment and HOME score. We are preparing a manuscript for publication on our Mn hair analyses.
|
|
7. Back-extrapolation of DDT/E and PBDEs: We evaluated three modeling approaches (physiologically-based pharmacokinetic modeling (PBPK), deletion substitution algorithms, and SuperLearner) to estimate maternal DDT/E and PBDE exposures during pregnancy when measurements were collected from the mothers nine years after birth. To accomplish this, we used the models to predict serum levels measured at 26 weeks gestation in the pregnant mothers (n=161) from levels measured in the mothers and the child when the child was 9. For each model, we assessed whether the nine-year maternal (n=94) or child (n=161) measurements alone, or levels in both mothers and children (n=89) at age nine, predicted the levels in the mothers during pregnancy. Model performance was assessed using the root mean squared error (RMSE) and coefficient of determination (R2) of log10-transformed back-extrapolated versus measured levels. For all compounds and subsets, SuperLearner outperformed the other approaches with RMSEs and R2s ranging from 0.10-0.31 log10 ng/g and 0.58-0.97, respectively. Typically, model RMSEs were lower and R2s were higher for p,p’-DDT/E than PBDE congeners, and estimations using maternal levels (when their child was 9 years) were more accurate for back extrapolation to pregnancy levels than using child levels at 9 years. The PBPK model performed well but not as well as SuperLearner when back-extrapolating prenatal levels from maternal levels for compounds with longer half-lives such as p,p’-DDE and BDE-153 (RMSEs= 0.21 and 0.28 log10 ng/g and R2s= 0.88 and 0.57, respectively). Overall, our results demonstrate the ability to accurately back-extrapolate prenatal levels from maternal levels 9 years after delivery, with SuperLearner performing the best based on our fit criteria. These findings were published in Environmental Science & Technology (Verner et al. 2015).
8. PBDEs and DDT/E exposure in 9-year-old boys: CDC analyzed CHAMACOS blood samples for PBDEs, DDT/E and PCBs in 412 mothers during pregnancy, 277 children at the 7-year visit, and 145 mothers and 552 children at the 9-year visit. The geometric means for these compounds decreased significantly (between 8 and 13 percent per year) in the children from age seven to nine years (Table B2). We also showed that BDE-153, the PCBs and DDE all decreased significantly per kilogram weight gain in the child related to dilution from an increasing body mass with age. The paper includes comparison of the CHAMACOS serum concentration data with a published cohort of Texas children (Sjodin et al. 2014). CHAMACOS participants had significantly higher exposures to PBDEs than children from Texas. Concentrations of PCB-153 were comparable in the CHAMACOS and Texas children while p,p’-DDE concentrations were higher in the primarily Mexican population of the CHAMCOS cohort. Dr. Andreas Sjödin published a manuscript examining determinants of PBDE and DDT/E exposures in 9-year-olds (Sjödin et al. 2018).
Table B2. Percentage change between age seven and nine by year and kilogram of weight gain for selected PBDEs, PCBs, DDT and DDE for participants with repeated measurements only.
9. OTHER CHAMACOS STUDIES
- Breastmilk (EPA Star Fellowship): We developed a new method to measure contemporary-use pesticides and persistent compounds. We collected breastmilk from women residing in the Salinas Valley and the San Francisco Bay Area (Weldon et al. 2011).
- PBDEs in Pregnant women and children (NIEHS): An article examining predictors of PBDES in maternal blood during pregnancy was published in ES &T (Castorina et al. 2011). An article was published in ES &T examining predictors of PBDE in child blood (Bradman et al. 2012).
- DAPs in the environment (EPA STAR Fellowship): A manuscript evaluating the contribution of preformed DAPs in the environment to DAPs in urine was published in JESEE (Quirós-Alcalá et al. 2012).
- BPA: An article on determinants of BPA exposure in pregnant women was published Environment International (Quirós-Alcalá et al. 2013). Two health outcome papers using BPA exposure data were published, one on children’s behavior (Harley et al. 2013) and another on adipokine levels in children (Volberg et al. 2013).
- Within and between subject variability: Dr. Bradman et al. (2013) published a paper in EHP examining within and between subject variability in organophosphate metabolite excretion in young children.
- Environmental Quality in Child Care: Dr. Bradman published a report examining environmental quality in child care with funding by the California Air Resources Board (Bradman et al. 2012).
- Organophosphate levels and PON1 in blood: Dr. Karen Huen (former graduate student with us) published a study reporting on chlorpyrifos and diazinon levels in maternal and infant blood and PON1 status (Huen et al. 2015).
- Dietary transitions in a Neanderthal infant: Dr. Manish Arora with Drs. Eskenazi and Bradman and other scientists, published a paper in Nature showing breast feeding transitions in a Neanderthal infant (Arora et al. 2013). The study used teeth and data from the CHAMACOS cohort to validate the method applied to a fossil Neanderthal tooth.
- DDT: Two papers were published on prenatal exposure to DDT/E and obesity in the CHAMACOS children (Warner et al. 2013; Warner et al. 2014).
- DAPs in child urine. A manuscript examining changes in pesticide excretion during organic food intake was published in Environmental Health Perspectives (Bradman, Quiros-Alcala et al. 2015).
- Occupational take-home Exposures: An article on reducing exposures to farmworkers’ children was published in the Journal of Environmental Epidemiology and Exposure Science (Salvatore et al. 2015).
Organophosphate flame retardant exposures and health impacts: We successfully obtained an NIEHS R21 (R21ES021833) and published manuscripts examining exposures (Castorina et al. 2017a) and health effects of organophosphate flame retardants (Castorina et al. 2017b).
Conclusions:
Our data collection work on this grant is complete, but we continue to work with data generated from this funding. We are currently preparing a manuscript for publication showing striking, sex-differentiated associations between prenatal OP pesticide exposures and children’s executive functioning and attention between ages 7 and 12. These indicate that boys experience pronounced deficits in maternally-reported executive function and child-assessed working memory in association with in utero OP exposure. We are also beginning work on analyses focused on the timing and tempo of puberty in CHAMACOS boys and girls, both as an outcome of early childhood adversity and as a predictor of risk-taking behavior in later adolescence. We expect to continue working with these data for years to come.
This portion of our Center grant set out to assess effects on boys’ neurodevelopment and pubertal development of prenatal and early life exposure to environmental exposures with wide U.S. and global relevance. We recognized that our Center was well poised to address societal concerns that environmental exposures could contribute to apparent increases in neurobehavioral issues like attention problems and a shifting age at puberty, and through our specific aims, we intended to do so. We have made important progress towards these aims. With regards to neurodevelopment, we have presented evidence of association between multiple early-life exposures and adverse neurobehavioral outcomes at this age point, particularly for boys. While prenatal PBDE exposure, prenatal methyl bromide and chloropicrin and early postnatal Mn exposure show similarly adverse associations with attention, executive functioning, and/or behavior for boys and girls, we provide evidence that prenatal Mn exposure and OP pesticide exposure (not yet published) may be particularly detrimental to boys. In so far as WISC Processing Speed and Working Memory Indices reflect attention and executive functioning capacities, respectively, our research suggests that prenatal DDT exposure and exposure to alternate flame retardants (Firemaster 500 or OP-based formulations) may also exert detrimental impacts on these outcomes. With regards to timing of puberty, we have provided evidence that PBDES, PCB, and DDT/E are associated with either earlier physical manifestations of male puberty or higher levels of male sex hormones at age 12. Early Mn exposure appears to be associated with slightly later genital development in boys, but was not associated with age 12 sex hormones (not published). In our parallel analyses of female pubertal timing, prenatal DDT exposure and early Mn exposure were associated with earlier menarche and earlier pubarche, respectively (not yet published). By contrast, prenatal PBDEs were associated with later menarche.
As noted above, the data collected under this grant and previous EPA funding have also enabled important research on other health outcomes of widespread interest in the U.S.: respiratory health and obesity. With regards to respiratory health, we observed associations between higher average urinary OP metabolite concentrations during childhood and increased respiratory symptoms and poorer lung function at 7-years of age. We also observed relationships between higher agricultural use of sulfur within 1 km of the child’s residence during the year prior to the 7-year visit and increased odds of reporting respiratory symptoms and poorer lung function. An important difference between the associations that we have observed with respiratory health and those with neurodevelopment is that postnatal pesticide exposures during early childhood appear to be more strongly related to adverse respiratory outcomes, whereas prenatal exposures consistently show stronger associations with neurodevelopmental outcomes. We did not observe any adverse relationship between prenatal or postnatal residential proximity to agricultural fumigant use and respiratory health in the CHAMACOS cohort. With regards to obesity, we’ve shown differential impacts of prenatal exposure to PBDEs and DDT/E based on child sex. Boys with higher prenatal exposure to PBDEs, DDT, and/or DDE all showed higher BMI in middle childhood, whereas girls with higher PBDE exposure tended to have a lower BMI in middle childhood (and showed no change in BMI linked to DDT/E exposure). One PBDE congener (BDE-153) as measured at age 7 was associated with lower BMI for both sexes at this age.
Novel epigenetic findings on the molecular mechanisms associated with environmental exposures in newborns and children resulting from the CHAMACOS Project C were only possible because of the existing CHAMACOS birth cohort data and banked biological samples collected over the children’s lifetime going back 18 years to the time when CHAMACOS children were in utero. This project required a careful examination of existing methodological and analytical approaches. We conducted a direct comparison of DNA methylation in different cell types that demonstrated that the commonly used minfi procedure may not be appropriate for newborns that have a different blood cell composition than adults. We developed novel methods of data normalization of high-dimensional genome-wide methylation data that was submitted to the public domain. We employed state-of the art analytical tools such as SuperLearner for statistical analyses and are now applying these methods in other studies.
Our data show striking differences in genome-wide DNA methylation by age and sex, not limited to CpG sites located in sex chromosomes. These findings provide additional evidence for the need to expand research on environmental epigenetics to a wide variety of sub-populations, including fetuses and pregnant women and minority cohorts (e.g. CHAMACOS) in order to explain differential risks and health effects. We first reported data from our birth cohort demonstrating that prenatal exposure to POPs such as PBDE and DDT that are still common in California and many other parts of the world and may be linked to hypomethylation in fetal blood. Importantly, we showed that accounting for co-exposure to different types of chemicals and adjusting for blood cell types may increase sensitivity of epigenetic analyses for epidemiological studies. These findings are immediately relevant to the EPA priority science question “What chemicals and combinations of chemicals… pose the greatest risk to children’s health”.
In short, within our Center, we have been able to trace important linkages between children’s early environmental exposures and multiple facets of their health and development in childhood. Beyond our Center, we’ve sought opportunities to contribute to collaborative investigations of these issues. In 2016, the CHAMACOS Study was selected to participate in the NIH-funded ECHO program (ECHO stands for Environmental influences on Child Health Outcomes), which has set out to construct a “synthetic cohort” of 50,000 US children by combining and harmonizing data collected from a large number of new and extant birth cohort studies around the country. Our participation in ECHO will facilitate use of CHAMACOS data – including data collected under this EPA funding – in any number of analyses related to children’s health and development.
The work completed as part of Project B of this Children’s Center grant advanced the field of exposure science in several ways. We evaluated a novel biomarker of perinatal Mn exposure by conducting detailed studies measuring Mn in dentine of children’s teeth and then characterizing prenatal and early postnatal sources of Mn exposure to evaluate dentine as a retrospective biomarker of exposure to Mn. This evaluation was only possible due to the wealth of stored environmental and biological samples available from the CHAMACOS cohort and the availability of Pesticide Use Data in California. In this first large-scale study of Mn exposure in children living in an agricultural region, we demonstrated that Mn in dentine was related to agricultural sources of Mn, including nearby agricultural use of Mn-fungicides using state of the art GIS modeling techniques, as well as Mn levels in house dust and the presence of agricultural workers living in the home. We were also able to show Mn levels in dentine provided estimates that were specific to the prenatal and early postnatal periods, which are critical time periods of development. We used stored house dust samples from the prenatal period to measure Mn concentrations and calculate Mn dust loadings that established home Mn contamination from agricultural sources as a likely pathway of exposure. We observed associations between nearby agricultural Mn-fungicide use, soil type at the residence and location of the residence and both concentrations and loading of Mn in house dust which not only identified Mn-fungicide use as a possible source of environmental Mn exposure, but further demonstrated the contamination of homes in agricultural communities from nearby pesticide use and the take-home pathway for farmworkerfamilies.
Our preliminary analyses of Mn concentrations in hair samples that were thoroughly cleaned prior to laboratory measurements indicate that this exposure biomarker is also related to nearby agricultural Mn-fungicide use and provides a promising non-invasive method of exposure assessment. In addition, in the course of this analysis, we have developed methodology for hair processing that will yield more valid measures of various exposures using this matrix.
With the benefit of stored prenatal blood samples that were used to evaluate prediction models, we developed novel methods to determine PBDE and DDT/E exposures during the prenatal period from blood samples collected 9-years later and identified important predictors of changes in blood levels of these compounds. Our analyses indicate that PBDE blood concentrations have declined in children after the removal of these compounds from consumer products in California. This study is important because it provides a basis for utilizing cross- sectional enrollment of children but allowing retrospective exposure assessment to improve the feasibility and statistical power of studies examining prenatal exposures and child health outcomes.
Journal Articles on this Report : 7 Displayed | Download in RIS Format
| Other subproject views: | All 108 publications | 43 publications in selected types | All 42 journal articles |
|---|---|---|---|
| Other center views: | All 697 publications | 170 publications in selected types | All 169 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental changes in autonomic nervous system resting and reactivity measures in Latino children from 6 to 60 months of age. Journal of Developmental and Behavioral Pediatrics 2011;32(9):668-677. |
R834513 (2012) R834513 (Final) R834513C001 (2012) R834513C002 (Final) R834513C003 (Final) |
Exit |
|
|
Alkon A, Boyce WT, Tran L, Harley KG, Neuhaus J, Eskenazi B. Prenatal adversities and Latino children's autonomic nervous system reactivity trajectories from 6 months to 5 years of age. PLoS One 2014;9(1):e86283. |
R834513 (2012) R834513 (2014) R834513 (Final) R834513C001 (2012) R834513C001 (2014) R834513C002 (Final) R834513C003 (Final) R826709 (2002) R831710 (Final) |
Exit Exit Exit |
|
|
Alkon A, Harley KG, Neilands TB, Tambellini K, Lustig RH, Boyce WT, Eskenazi B. Latino children's body mass index at 2-3.5 years predicts sympathetic nervous system activity at 5 years. Childhood Obesity 2014;10(3):214-224. |
R834513 (2012) R834513 (2014) R834513C001 (2012) R834513C001 (2014) R834513C002 (Final) R834513C003 (Final) R826709 (2002) |
Exit Exit Exit |
|
|
Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, Smith DR. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environmental Science & Technology 2012;46(9):5118-5125. |
R834513 (2010) R834513 (2012) R834513 (2013) R834513 (2014) R834513 (Final) R834513C001 (2012) R834513C002 (2012) R834513C002 (2013) R834513C002 (Final) R834513C003 (Final) |
Exit Exit Exit |
|
|
Audelo J, Kogut K, Harley KG, Rosas LG, Stein L, Eskenazi B. Maternal depression and childhood overweight in the CHAMACOS Study of Mexican-American children. Maternal and Child Health Journal 2016;20(7):1405-1414. |
R834513 (2014) R834513 (2015) R834513 (2016) R834513 (Final) R834513C001 (2014) R834513C001 (2015) R834513C001 (2016) R834513C002 (Final) R834513C003 (Final) |
Exit Exit |
|
|
Beamer PI, Canales RA, Ferguson AC, Leckie JO, Bradman A. Relative pesticide and exposure route contribution to aggregate and cumulative dose in young farmworker children. International Journal of Environmental Research and Public Health 2012;9(1):73-96. |
R834513 (2013) R834513 (Final) R834513C002 (2013) R834513C002 (Final) R834513C003 (Final) |
Exit Exit Exit |
|
|
Harley KG, Engel SM, Vedar MG, Eskenazi B, Whyatt RM, Lanphear BP, Bradman A, Rauh VA, Yolton K, Hornung RW, Wetmur JG, Chen J, Holland NT, Barr DB, Perera FP, Wolff MS. Prenatal exposure to organophosphate pesticides and fetal growth: pooled results from four longitudinal birth cohort studies. Environmental Health Perspectives 2016;124(7):1084-1092. |
R834513 (2014) R834513 (2015) R834513 (2016) R834513 (Final) R834513C001 (2014) R834513C002 (2014) R834513C002 (Final) R834513C003 (Final) |
|
Supplemental Keywords:
Health, RFA, Scientific Discipline, INTERNATIONAL COOPERATION, Health Risk Assessment, Environmental Policy, Biology, Children's Health, biological markers, harmful environmental agents, pesticide exposure, agricultural community, flame retardants, neurochemical effects, PBDE, children's vulnerablity, farmworkersProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R834513 Center for Research on Early Childhood Exposure and Development in Puerto Rico Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R834513C001 CHAMACOS Cohort Project: Pesticides and PBDE on Neurobehavior and Puberty
R834513C002 Project B: Exposure Project: Mn, DDT/E and PBDE Exposure to Farmworker Children
R834513C003 Epigenetics Project
R834513C004 Community Outreach and Translation Core
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2016 Progress Report
- 2015 Progress Report
- 2014 Progress Report
- 2013 Progress Report
- 2012 Progress Report
- 2011 Progress Report
- 2010 Progress Report
- Original Abstract
42 journal articles for this subproject
Main Center: R834513
697 publications for this center
169 journal articles for this center