Grantee Research Project Results
2009 Progress Report: Metal Mixtures and Children’s Health
EPA Grant Number: R831725Center: Health Effects Institute (2015 - 2020)
Center Director: Greenbaum, Daniel S.
Title: Metal Mixtures and Children’s Health
Investigators: Hu, Howard , Brain, Joseph D.
Current Investigators: Hu, Howard , Brain, Joseph D. , Wright, Robert , Adams, Jeff , Cohen, Amy , Schaider, Laurel , Weisskopf, Marc , Bellinger, David , Hatley, Earl , Wright, Rosalind , Peterson, Karen E. , Shine, James P. , Wessling-Resnick, Marianne , Molina, Ramon , Maher, Tim , Spengler, John D. , Schwartz, Joel , Backus, Ann
Institution: Harvard University
EPA Project Officer: Callan, Richard
Project Period: June 1, 2004 through May 31, 2009 (Extended to May 31, 2011)
Project Period Covered by this Report: June 1, 2008 through May 31,2009
Project Amount: $7,894,185
RFA: Centers for Children's Environmental Health and Disease Prevention Research (2003) RFA Text | Recipients Lists
Research Category: Children's Health , Human Health
Objective:
The Center for Children's Environmental Health and Disease Prevention at the Harvard School of Public Health has just completed its fifth year. The final year of funding ended March 30, 2008. As of April 1, 2009, our Center continues, supported by several other grants, and especially by a no cost extension.
More and more, our Center has become a model of effective translational research. Our Center uses animal models to address fundamental mechanisms of metal pharmacokinetics and mechanisms of injury. It utilizes exposure measurements in humans, and also measures health outcomes in humans and animals. Most importantly, we involve the community. We have established effective lines of communication with individuals in the Tar Creek area, as well as with government stake-holders.
The community that forms our Children's Center, both in Boston and in Oklahoma, is eager to continue. We are convinced that our partnership is mutually productive and is making important contributions to science relevant to policy formation in relation to the health of children. In addition to maintaining some activities with our no cost extension, Dr. Robert Wright has taken the lead in crafting a competing renewal. Moreover, we envision several adjacent grants which will further develop some of our key themes. Dr. Bellinger obtained an R01 (ES016283-01) entitled "Metal Exposure and Children's Preschool Neurodevelopment." Drs. Wessling-Resnick and Brain submitted an NIH Challenge Grant focusing on metal mixtures and neurobehavioral outcomes – another extension of Children's Center themes.
Progress Summary:
Year 05 was marked by substantial progress in all Projects and Cores. Let us begin by discussing Project 1 entitled "Metals, Nutrition, and Stress in Child Development." This project is slowly recovering from a series of weather-related events which heavily impacted study activities. Following the flood and ice storms of 2007, there was an ice storm in February 2008. Then, in the summer of 2008, a tornado hit Picher, OK, and destroyed many homes as well as displaced some of a chat pile. A relative of one of our field staff workers died in this tornado. Generally, many study families as well as our staff were affected by these series of disasters.
We were able to reorganize by July 2008, and in the current grant year have recruited more than 80 new mothers. With the support of the new R01 mentioned earlier, the birth cohort established by the Center will continue until 2013. Recruitment will continue and we expect to increase the overall sample size to 600 children. The new R01 will follow subjects until age 6. In addition, because not all subjects will be six years old by 2006, we will see all subjects recruited in the coming year at birth and at age 4 years. We will conduct the intelligence tests that have been planned to test whether blood manganese and material stress influence Bayley scales.
Project 2 is entitled "Exposure Assessment of Children and Metals and Mining Waste: Composition, Environmental Transport, and Exposure Patterns." This project was also impacted by the flooding and other weather-related events which impacted field research in the Tar Creek area. In fact, the flood presented an opportunity to examine metal exposures caused by the deposition of sediments from Tar Creek and the Neosho River. This natural disaster allowed us to develop and deploy a geochemical fingerprint approach for different metal sources. Data were collected to examine the mixing that occurred during the flood and the resulting concentration patterns. We have also been studying mine waste pile runoff and seepage from underground mines. We have quantified the extent of metal transport into the Tar Creek watershed and how it relates to both active and inactive mines.
As part of our ongoing collaborations with Project 3, we are assessing the bioaccessibility and bioavailability of Zn present in various mineralogical forms. We obtained samples of 5 zinc minerals from the Mineralogical Collection at Harvard's Museum of Natural History. Two of these minerals, sphalerite (ZnS) and hemimorphite (Zn silicate), were primary minerals present at the Tar Creek site. The mineralogical samples were pulverized and sieved to <37 μm. These fine-grained samples, in addition to a <37 μm chat sample, were neutron activated (NA) at MIT and aliquots were used in both in vivo bioavailability studies in rats and in vitro bioaccessibility tests using the Simple Bioaccessibility Extraction Test (SBET), a simulated gastric fluid extraction.
As predicted, our results showed that sphalerite was the least bioaccessible form of Zn, which was consistent with previous studies showing that metal sulfides have low bioavailability. Similarly, in vivo Zn absorption studies in rats showed that sphalerite had the lowest bioavailability of all the minerals tested. We also expected that smithsonite (ZnCO3) would have high bioaccessibility, since metal carbonates are thought to be highly bioavailable. While the bioaccessibility of smithsonite was substantially higher than that of sphalerite, the other three minerals all had even higher bioaccessibility than smithsonite. For all samples, the bioaccessibility as measured using NA samples was higher than those measured using ICP-MS. We will determine whether these differences are due to the different solid:liquid ratio used, to the NA process and resulting pool of potentially leachable metals, or to the methods of determining the total amount of Zn present. For instance, the "total" Zn in the NA samples was determined directly by measuring 65Zn in the samples prior to extraction, whereas the "total" Zn in the non-NA samples was based on theoretical values, which could underestimate bioaccessibility if there are impurities in the minerals.
Project 3 is utilizing a pregnant rat model to better understand metal exposures of children and their mothers in contaminated settings like Tar Creek by (1) utilizing exposures during and after pregnancy and lactation, (2) using metal ions as well as complex environmental samples from Tar Creek, and (3) comparing different routes of entry from the environment into the body. Rats are being exposed during gestation, lactation, and after weaning by intratracheal instillation, by gavage, via intranasal administration, or by intravenous injection. Our data clearly shows that the absorbed dose of metals in critical organs such as the brain depends on route of entry and duration of exposure. They also indicate that uptake from the nose and the lungs may be underappreciated in contrast to ingestion. Analyses of these data will be used to help estimate the relative risks of metals from different exposures, e.g. eating contaminated food and water, inhaling airborne chat particles, or children playing in contaminated playgrounds. Adjacent studies have helped us understand molecular mechanisms of metal transport in both normal and mutant rats, and to elucidate mechanistic differences between manganese and iron absorption.
As mentioned earlier, a family of zinc minerals was characterized in cooperation with Project 2. Our studies with neutron-activated particles demonstrated a good correlation between the surface area of particles, as determined by nitrogen adsorption and in vivo bioavailability results. The greater the surface area per gram, the greater the zinc bioavailability. We also continued our studies on chat particles and employed elemental analysis by neutron-activation to examine the transport and retention of specific chat-associated metals – primarily 59Fe or 65Zn. We continue to be interested in mechanisms of manganese and iron absorption from the lungs and nose and the influence of iron status on iron absorptive kinetics. We studied olfactory absorption of 54Mn in Belgrade rats, an animal model of DMT1 deficiency, and established that transport of intranasally instilled metal from the nasal cavity to the blood was impaired. Moreover, this pathway of manganese absorption was enhanced in anemic rats relative to iron-sufficient controls. These findings are significant because they suggest that neurotoxicity of inhaled manganese may be modified by iron status.
A final series of experiments, carried out in collaboration with Project 4, studied exposure to manganese (Mn) and lead (Pb) during early development. We showed that exposures during this time may be related to impaired learning and cognitive functions, as well as higher distractibility and impulsiveness, including attention deficit hyperactivity disorder. Manganese exposed pups (but not lead exposed) were more impulsive than control pups. Both manganese and lead exposed pups displayed lower overall velocities of movement when compared with control pups. We also observed that pups from dams given manganese (Mn) or lead (Pb) had significantly lower body weight and higher blood and brain concentrations of the respective metal. These data will be useful in assessing the relative risks for metal toxicity of various exposures to metals. The pharmacokinetic results from chat studies show differences in absorption, vascular kinetics and tissue retention of 59Fe or 65Zn from irradiated chat administered via different routes in rats. Significant differences were also observed as a function of particle size. Smaller, respirable chat had higher metal bioavailability when inhaled or ingested. Data from these studies will be used to assist in estimating the relative risks of metals from different exposures, e.g., eating contaminated food and water, inhaling airborne chat particles, or children playing in contaminated playgrounds.
Project 4 has utilized exposures to metals, especially by ingestion, and has focused on behavioral and neurochemical outcomes. Changes in body weight and other health and disease indicators have also been seen. This project utilizes exposures via drinking water. Project 4 emphasizes the long term consequences of perinatal exposure to different concentrations of manganese and lead. Emphasized are behavioral changes and changes in neurochemistry. Project 4 worked closely with Core B (Analytical Chemistry) to quantify levels of heavy metals in the blood and brain of the offspring of exposed mothers. Increases in brain levels of lead and manganese was dose related and was demonstrable in multiple brain tissues, including cortex, hippocampus, and brain stem. The Morris Water Maze and the elevated-plus maze (EPM) were performed in rats to assess impulsivity and hyperactivity. These results provide evidence that exposure to manganese chloride and lead acetate during gestation and lactation cause subtle neurobehavioral and neurochemical changes resulting from a wide range of exposure levels. These results provide the evidence that exposure to MnCl2 and Pb acetate during gestation and lactation causes subtle neurobehavioral and neurochemical changes resulting from a wide range of the exposure levels. The data also emphasized the significance of the low doses of MnCl2 and Pb acetate exposures, as impulsivity and hyperactivity were observed from both male and female rats with Pb acetate 2.5-25 μg/ml (blood Pb level lower than 10 μg/dL). In contrast, these neurobehavioral deficits were not shown from high concentration of Pb acetate (2500-4000 μg/ml) that produced very high Pb level in blood and brain tissues. Additionally, low doses of MnCl2 of 1.25 and 5 mg/ml, but not 10 mg/ml produced hyperactivity. However, we can not conclude that these neurobehavioral deficits did not occur with the high levels of MnCl2 and Pb acetate, as other toxic effects resulting from high level exposures can obscure the impulsivity and hyperactivity. Learning and memory were also affected from MnCl2 and Pb acetate exposures. Learning and memory impairment was shown from the medium to high level exposures of Pb acetate (Pb acetate of 100-250 μg/ml and 2500-4000 μg/ml), and MnCl2 of each level (MnCl2 1.25, 5 and 10 mg/ml).
In addition to the neurobehaviors, the results from the microdialysis experiments demonstrate the neurochemical changes from the exposure to these metals. Both dopaminergic and glutamatergic neurotransmissions were affected from MnCl2 exposure, although a gender effect was apparent. Exposure to MnCl2 10 mg/ml in male and female rats had a smaller increase of K+ evoked glutamate levels than controls. This deficit might relate to the learning and memory impairment observed in male and female rats exposed to higher MnCl2 levels. In contrast, there was a gender effect of MnCl2 exposure on the basal and K+ evoked DA levels. The deficit was found only in female rats as evidenced by higher basal DA and decreased K+ evoked DA levels. That the deficit in dopaminergic neurotransmission was found only in female rats might be related to the hyperactivity found from MnCl2 1.25 and 5 mg/ml in female rats.
A very successful third meeting of the External Advisory Committee was held in Tar Creek, Oklahoma during our fourth year. This provided us with an opportunity to hear from the community we are studying, as well as gave us a chance to communicate our preliminary findings and advice about reducing risk. We recommend this to other Centers as a way of connecting more effectively with participating communities. We continue to maintain a monthly schedule of Project and Core meetings. The Chemistry Core is processing an increasing number of samples, especially for Project 2. A new apparatus is now being used to aerosolize chat samples, thus creating an opportunity to collect and characterize respirable particles, some of which will be neutron-activated by Project 3. The Biostatistics Core has been involved with multiple publications and continues to develop new analytic methods as needed. Increasingly, we are expanding our talent in regard to geographic information systems (GIS). Spatial analysis is emerging as an important tool. The Community Outreach and Translation Core is active. A comic book has been produced for use in Tar Creek. The Community Outreach and Translation Core has been unusually active and successful. Finally, the Administrative Core has fulfilled its role to implement its quality management plan.
Core A: Administration
Core Leader: Joseph Brain
Core Co-Leader: Robert Wright
During the past year, our Centers, like others, have adapted to the hiatus in funding caused by the delays in the Children's Center RFA being released. In spite of this, we've done our best to maintain our momentum and to plan for the future. Dr. Robert Wright will take over as the Director of our Children's Center, and has taken the lead in submitting a competing renewal. Adjacent to this is the planning and submission of multiple R01s and other grants, which will maintain our integrated and highly productive effort, focusing on metal exposures and neurobehavioral outcomes.
This year also was marked by continued reductions in exposures to toxic metals in Tar Creek. It is being increasingly documented by this Children's Center, as well as by other local, state, and federal agencies. Last year, our colleague, Rebecca Jim, in Tar Creek published a book sponsored by the LEAD Agency in Vinita, Oklahoma entitled "Making a Difference at the Tar Creek Superfund Site: Community Efforts to Reduce Risk." This is a compelling story of the past, present, and future of this Superfund site, well documented with personal stories and photographs. This year, Rebecca Jim edited another illustrated volume entitled "Disasters: Flood & Ice." This book documents with personal stories, photos, and drawings of adults and children the impact of the multiple disasters on the Tar Creek community and its interaction with mining wastes.
The Administrative Core continued to operate and update our website (http://www.hsph.harvard.edu/niehs/children ![]() ) that provides key information on each of the Projects and Cores and that has been used for updates on events, publications, and other developments.
) that provides key information on each of the Projects and Cores and that has been used for updates on events, publications, and other developments.
Another activity of Core A is planning an international effort focusing on the prevention and mitigation of the environmental and health consequences of metal mining and smelting. This is being planned in conjunction with Dr. Mary Jean Brown at the CDC. We are developing a web of collaborators throughout the United States and in other countries who are working on this problem. Our vision is to emphasize the prevention of Superfund Sites. What global regulations should be in place that would make it less likely that metal mining will have persistent negative impacts.
Quality Assurance
The Center Quality Management Plan (QMP) requires QAPPs to be prepared for all research related activities and that an annual quality improvement audit be conducted for each research project. All QAPP's for all four projects are on file. During the period from September through October 2007, Jose Vallarino, the Center's Quality Assurance Officer, conducted quality improvement audits on all four research projects. The purpose of the audits was to assess whether all quality-related documents (Written Standard Operating Procedures, log sheets, logbooks and other required records were readily available and complete.)
Core B: Analytical Chemistry
Core Leader: John Spengler
Core Co-Leader: Jim Shine
The Analytical Chemistry Core has two components: the trace metal facilities and development of sampling equipment. The trace metal facilities are maintained to serve the analysis needs of the various projects. Trace metal analyses of blood, hair and urine samples collected at Tar Creek, OK continue. Last year's progress report summarized the levels of Pb, Mn and Cd measured in maternal and infant blood, as well as Pb, Mn, Cd and As measured in hair of mothers and newborns, and pilot studies on meconium and saliva. This report focuses on new developments. Reported here are the summaries of two recently initiated pilot studies involving our Tar Creek birth cohort. These are studies of metals in meconium and breast milk.
While the core did not develop new sampling equipment this year, we did purchase (with non-Center funds) a Niton XL3 Portable XRF device. After training and registering the use of an X-ray source in Oklahoma, this device was used in Tar Creek to survey surface soil for chat-related metals following tornadoes that ripped through the Pitcher, OK on May 10, 2008 to examine the extent that mine waste was further distributed throughout the community.
The core continues to provide training to visiting scientists and students on metal analyses. The core has made the Niton XRF device available to several students to explore metals in road dust, toys, house dust, paint and garden soils.
New Exposure Assessment
i. Metals in Meconium:
In 2007, the trace metals facility began developing methods for analyzing meconium (first postnatal stool) samples collected from birth cohort infants as part of Project 1. These methods were finalized in 2008, and the application of the analysis methods to samples collected at the Tar Creek Superfund Site has begun. In total, 124 samples were collected: 59 from infants enrolled in the cohort study, and 65 from infants not enrolled in the study. These non-cohort samples will be used to refine the sample analysis methodology and to give researchers a better sense of the distribution of metals levels found in meconium in the larger population.
Field staff collected each diaper containing meconium from all infants who participated in either the cohort or the non-cohort arm of the study. For the majority of infants, meconium from each diaper will be combined to create one large "pooled" sample. For a subset of infants, meconium from each diaper will be processed separately to allow us to detect any trends in meconium metal concentration over the pregnancy (it has been theorized that serial meconium samples reflect different time periods during gestation, with meconium samples excreted earliest representing earlier time periods and meconium samples excreted later representing later time periods).
Methods for each stage of sample preparation and analysis were tested in the first part of 2008. The digestion step in particular proved difficult to refine, but as of September 2008 all methods were finalized, with standard operating procedures in place. Samples are first transferred from the diaper into a trace-metal free container and freeze-dried for 3 days. After drying, samples are homogenized using a Spex SamplePrep Mixer/Mill and acid-digested in concentrated nitric acid using a microwave oven digestion method. Samples are analyzed using ICP/MS. Urine contamination of meconium samples appears to be common, and chloride ions in the urine can create an interference for arsenic when combined with the argon plasma in the ICP/MS instrument. Therefore, samples will be analyzed for As separately using the Dynamic Reaction Cell, which eliminates ArCl interference for As.
To date, 9 pooled samples from 9 infants and 34 serial samples from 10 infants have been analyzed for Pb, Cd, and As. Manganese analysis was delayed by a software problem which was discovered and fixed in November 2008; results are forthcoming.
Table C2.1 shows the results of our analyses thus far. Samples which were analyzed "serially" (where multiple samples were analyzed separately, instead of pooled, for each infant) have been mass-weighted and averaged to produce a good estimate of the metal concentrations for each infant, had we pooled the serial samples prior to analysis. These mass-weighted results (N=10 infants and 34 samples) have been combined with the results of the pooled sample analysis (N=9 infants and 9 samples) for presentation.
Table C2.1: Metal content in pooled meconium samples. Concentrations for serial samples were mass-weighted and averaged to produce an accurate estimate of a pooled sample. Results are presented only for values greater than the limit of detection. Concentrations are presented in nanograms of metal per gram of dry meconium (parts per billion).
|
Percent detect
|
Mean (SD)
|
Minimum
|
Maximum
|
|
|---|---|---|---|---|
| Lead |
74%
|
13.1(7.2)
|
1.05
|
27.9
|
| Cadmium |
37%
|
8.39(19.7)
|
0.65
|
53.0
|
| Arsenic |
95%
|
28.3(18.1)
|
6.73
|
73.5
|
Detectible levels of lead, cadmium, and arsenic were all in the range of 1 to 100 parts per billion. These levels are much lower than those reported in the few other studies that have presented analyses of metals in meconium (Ostrea et al., 2002; Turker et al., 2006). This may be due to lower exposure levels in our group, or to the greater sensitivity of the ICP/MS for detecting very low concentrations, or to some combination of the two.
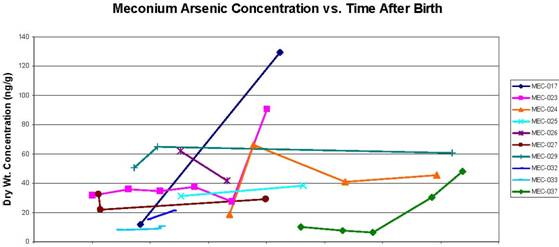
Figures C2.1 through C2.3 show the results of the "serial" analysis of 10 infants' samples. Each figure presents meconium metal concentration vs. the time when the sample was collected, in hours postpartum. These figures suggest that metal concentration in meconium does increase in samples collected later in the postpartum window. The significance of this finding is unclear, but certainly warrants further exploration, as another study has also found increasing levels of environmental contaminants in serial meconium samples with increasing time from birth (Ortega García et al., 2006). Although the significance of these trends in serial meconium samples remains somewhat unclear, it is possible that serial samples represent different time periods in the pregnancy, and therefore different time windows for exposure. Increasing concentrations of environmental contaminants in serial meconium samples could therefore indicate higher exposures to the fetus later in the pregnancy. However, they could also indicate an increase in maternal-to-fetal transfer of these contaminants later in the pregnancy, or some other unexplored mechanism.
It is important to emphasize that these results are quite preliminary, and are based on analysis of a small percentage of the collected samples. Results are likely to change substantially as the remaining samples are analyzed.
Figure C2.1. Concentration of lead in serial meconium samples vs. the number of hours after birth that the sample was collected. For samples below the limit of detection, a value of half the limit of detection was applied. Concentrations are presented in nanograms of metal per gram of dry meconium (parts per billion).
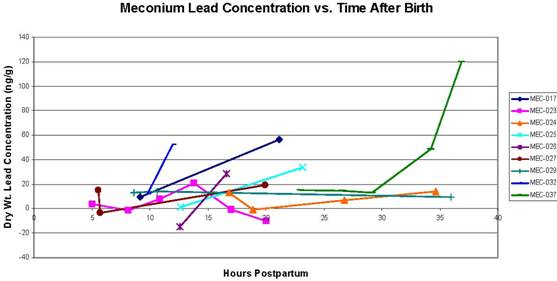
Figure C2.2. Concentration of cadmium in serial meconium samples vs. the number of hours after birth that the sample was collected. For samples below the limit of detection, a value of half the limit of detection was applied. Concentrations are presented in nanograms of metal per gram of dry meconium (parts per billion).
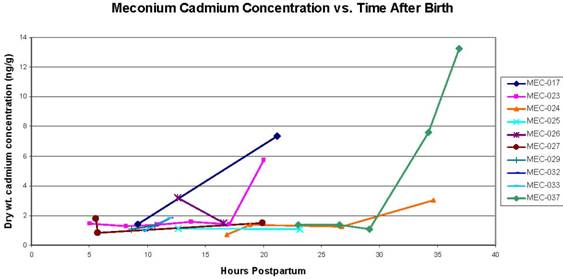
Figure C2.3. Concentration of arsenic in serial meconium samples vs. the number of hours after birth that the sample was collected. For samples below the limit of detection, a value of half the limit of detection was applied. Concentrations are presented in nanograms of metal per gram of dry meconium (parts per billion).
ii. Metals in Breast Milk and Formula
With support of NIEHS center pilot grant we are planning to study "Metals in Breast Milk and Infant Formula – Exposures and a Potential New Biomarker of Early Biologic Effects in Infants Born near a Superfund Site". This project evaluates human exposure to metals in breast milk and formula among infants from communities surrounding the Tar Creek Superfund site. Concentrations of Pb, Mn, As, Cd, Zn, Fe and Cu in breast milk of lactating mothers will be measured at four time points: in the early postpartum period (up to 2 days after delivery), and at 1, 3, and 6 months postpartum. Since it is anticipated that not all mothers will be exclusively breastfeeding their infants, formula (as prepared for the infant) will also be collected. The breast milk (and formula) metals concentrations will then be used, in combination with information on infant feeding practices, to predict metals biomarker concentrations in blood, hair, nails, urine) from birth to 12 months of age in order to evaluate the contribution of infant feeding as a pathway of exposure. Finally, a secondary aim is to evaluate the modifying influence of metals exposure during pregnancy on the relationship between maternal plasma prolactin levels (measured at delivery) and the lactational transfer of metals into breast milk. Given the known influence of metals on maternal plasma prolactin levels, we will also investigate if infant plasma prolactin levels (measured in umbilical cord blood at delivery) are sensitive to metals exposure. A future study will address the potential for adverse health effects from breast milk through lactational transfer of toxicants. Preliminary data analyzed from this pilot study will allow us to clarify our specific aims related to prolactin as a new biomarker for reproductive toxicity of metals.
To date, we have developed environmental sampling protocols and begun field collection of breast milk and formula from our cohort. A total of 14 breast milk and 53 formula samples have been collected from 50 women. We have undertaken laboratory methods development for milk analysis of metals other than lead. After testing several sample preparation techniques we have validated the microwave digestion method as the method for analyzing multielements (Lead (Pb), Cadmium (Cd), Manganese (Mn), Molybdenum (Mo), Copper (Cu) and Arsenic (As), in breast milk and formula samples. Since Mo interferes with Cd analysis (most of the Cd isotopes have interference due to formation of MoO+ molecular ion) samples were also analyzed for molybdenum to see whether significant amount of Mo present in breast milk samples. Experiments confirmed that low concentration of Mo present in breast milk samples doesn't create problem on Cd measurement. Mercury in breast milk and formula samples will be analyzed directly (without sample preparation) using direct mercury analyzer (DMA-80, Milestone). We have completed the analysis of all the breast milk samples (14) currently collected and in the process of analyzing 53 formula samples. Results for the breast milk samples are given in the Tables C2.2 and C2.3.
Table C2.2. Multielement (Pb,Cd and Mn) Analysis of Breast Milk Samples
| Subject-ID | Infant's age | Pb | Pb-SD | Cd | Cd-SD | Mn | Mn-SD | |
|---|---|---|---|---|---|---|---|---|
| ng/ml | ng/ml | ng/ml | ||||||
|
BM1 |
9 months |
0.60 |
0.03 |
0.08 |
0.03 |
5.71 |
0.16 |
|
|
BM2 |
7 Months |
4.61 |
0.26 |
0.10 |
0.03 |
3.32 |
0.11 |
|
|
BM3 |
4 months |
0.40 |
0.03 |
< dL |
|
2.11 |
0.11 |
|
|
BM4 |
3 months |
1.91 |
0.09 |
< dL |
|
2.42 |
0.04 |
|
|
BM5-S1 |
1 month |
0.24 |
0.01 |
< dL |
|
1.36 |
0.04 |
|
|
BM5-S2 |
3 months |
0.26 |
0.02 |
< dL |
|
1.14 |
0.06 |
|
|
BM5-S3 |
4 months |
0.24 |
0.03 |
< dL |
|
1.14 |
0.04 |
|
|
BM6 |
4 months |
0.26 |
0.03 |
< dL |
|
0.69 |
0.04 |
|
|
BM7 |
4 months |
0.48 |
0.03 |
< dL |
|
2.05 |
0.04 |
|
|
BM8-S1 |
1 months |
0.33 |
0.03 |
0.08 |
0.01 |
1.96 |
0.05 |
|
|
BM8-S2 |
5 month |
0.43 |
0.06 |
0.09 |
0.03 |
1.82 |
0.09 |
|
|
BM9 |
1 month |
0.60 |
0.11 |
0.07 |
0.01 |
-0.67 |
0.02 |
|
|
BM10 |
6 months |
0.96 |
0.20 |
< dL |
|
6.14 |
0.08 |
|
|
BM11 |
7 months |
1.19 |
0.06 |
0.14 |
0.05 |
2.64 |
0.09 |
|
|
dL=0.01 ng/g |
||||||||
Table C2.3. Multielement (Mo,As and Hg) analysis of Breast Milk Samples
| Subject-ID | Infant's age | Mo | Mo-SD | As – Milk | As-SD | Hg | Hg-SD |
|---|---|---|---|---|---|---|---|
| ng/ml | ng/ml | ng/ml | |||||
|
BM1 |
9 months |
1.31 |
0.06 |
0.17 |
0.47 |
0.26 |
0.03 |
|
BM2 |
7 Months |
0.58 |
0.07 |
|
0.11 |
0.26 |
0.03 |
|
BM3 |
4 months |
< dL |
|
|
0.09 |
0.16 |
0.02 |
|
BM4 |
3 months |
1.08 |
0.10 |
0.50 |
0.17 |
0.27 |
0.03 |
|
BM5-S1 |
1 month |
0.48 |
0.04 |
|
0.28 |
0.32 |
0.04 |
|
BM5-S2 |
3 months |
0.32 |
0.04 |
|
0.13 |
0.25 |
0.03 |
|
BM5-S3 |
4 months |
0.26 |
0.03 |
|
0.13 |
< dL |
|
|
BM6 |
4 months |
< dL |
|
|
0.29 |
0.16 |
0.02 |
|
BM7 |
4 months |
< dL |
|
|
0.12 |
0.24 |
0.03 |
|
BM8-S1 |
1 months |
0.24 |
0.07 |
|
0.20 |
< dL |
|
|
BM8-S2 |
5 month |
0.40 |
0.02 |
|
0.11 |
< dL |
|
|
BM9 |
1 month |
-0.27 |
0.02 |
|
0.07 |
0.43 |
0.05 |
|
BM10 |
6 months |
2.38 |
0.14 |
0.56 |
0.16 |
0.25 |
0.03 |
|
BM11 |
7 months |
0.50 |
0.08 |
|
0.75 |
0.23 |
0.03 |
|
dL=0.01 ng/g |
|||||||
Below is a summary of all the laboratory analyses done by the Analytical Chemistry Core in 2008.
Table C2.4 Children Center related sample analysis for 2008
| Sample type | Elements | # of samples analyzed |
|---|---|---|
|
Mothers-Blood |
Pb,Mn,Cd |
55 |
|
Child-blood |
Pb,Mn,Cd |
46 |
|
Mothers-Blood |
As |
570 |
|
Breast Milk |
Pb,Cd,Mn,Mo,As,Hg |
14 |
|
Rat-Blood |
Pb,Mn |
113 |
|
Rat-Brain (sections) |
Pb,Mn |
194 |
|
Rat-Blood |
As |
20 |
|
Rat Brain |
As |
84 |
|
Total samples analyzed |
|
1096 |
Training
The core has provided opportunities for visiting scientists to work with researchers on trace metal analysis methods. Further the Analytical Chemistry Core has trained several students in field and laboratory techniques for quantifying metal concentrations in environmental and biological sampling.
The availability of a portable XRF instrument this year has led to more students conducting projects exploring metals in the environment.
This section presents summary of the training provided by Core B is presented in Table C2.5
Table C2.5 Trainees working with the Analytical Chemistry Core
| Name | Affiliation | Approximate Dates | Description of Activities |
|---|---|---|---|
|
Visiting Scientists |
|||
|
Dr. Innocent Jayawardene |
Fulbright Fellow Univ. of Sri Jayewardenepura, Sri Lanka |
10/2006-07/2009 |
Method development and sample analysis - Multielement analysis of breast milk samples |
|
Dr. James Hauri |
Dept of Natural Sciences |
2008-2009 |
Meconium development method |
|
Undergraduate Students |
|||
|
Kathleen McCarthy |
Sr. Thesis, Wellesley College |
2005-2009 |
Field sampling and laboratory analyses |
|
HSPH Doctoral Studenst/Graduates |
|||
|
Eric Apeagyei |
Harvard School of Public Health |
08/2008-12/2008 |
Testing car tires for trace metals; |
|
Rebecca Lincoln |
Harvard School of Public Health |
January 2006-2009 |
Assisted in the collection of samples from participants’ home environments; designed and implemented a protocol for collecting meconium samples from study infants; assisted in the development of an analytic method for detecting metals in meconium. |
|
Ami Zota |
Harvard School of Public Health |
2005-2008 |
Analyzed samples of household dust and drinking water for metal content using the ICP-MS |
|
Others |
|||
|
David Mascarito |
Harvard University Extension School |
09/2008-12/2008 |
Testing for trace metals in road dust from Greenfield, MA |
|
Derrick Brain |
Harvard University Extension School |
09/2008-12/2008 |
Testing for testing for trace metals in road dust from I-93 ( Somerville, MA) |
References
Ortega García JA, Carrizo Gallardo D, Ferris i Tortajada J, García MM, Grimalt JO. 2006. Meconium and neurotoxicants: searching for a prenatal exposure timing. Arch Dis Child, 91(8):642-6.
Ostrea EM, Morales V, Ngoumgna E, Prescilla R, Tan E, Hernandez E, Ramirez GB, Cifra HL, Manlapaz ML. 2002. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. Neurotoxicology, 23(3):329-39.
Turker G, Ergen K, Karakoç Y, Arisoy AE, Barutcu UB. 2006. Concentrations of toxic metals and trace elements in the meconium of newborns from an industrial city. Biol Neonate, 89(4):244-50.
Core C: Biostatistics
Core Leader: Joel Schwartz
Data Coordination and Statistical Core. Dr. Joel Schwartz is the Principal Investigator of this Core, with Ms. Tania Kotlov (who has coordinated data management for large studies for >20 years) supervising Data Coordination. Dr. Brent Coull and Dr. Antonella Zanobetti are co-investigators. Ms Kotlov continues to actively supervise the collection of the data, as well as prepare our major databases. As part of this effort, she has frequent contacts with field staff in Tar Creek, and made a week long visit during the last year to assure data quality. Members of this core interact with each other on the projects in both the design of sampling frames and in the conduct of the analysis. They also continue to serve on the doctoral committees of students involved in analyzing the data, providing hands on guidance on statistical issues. This involves exposure as well as epidemiologic studies. In addition, Dr. McCracken has aided with design and analysis issues for animal studies.
Core D: Community Outreach and Translation
Core Leader: Rebecca Jim
Core Co-Leaders: Earl Hatley, Howard Hu, and Ann Backus
The Last Five: For five years, from 2003-2008, Harvard School of Public Health researchers have undertaken studies of heavy metal exposures in mother/infant pairs and of the fate, transport, deposition, and bioavailability of metals associated with the Tar Creek Superfund site located in Ottawa County, Okahoma. Additional studies in animal models of routes, target organs, and behavioral outcomes of exposure have taken place in parallel with the human and field studies. Interaction with the community has been critical and constant during this time. A Community Advisory Board (CAB), into which was incorporated the Tribal Sub-Committee, has been the sounding board for a number of methodological questions such as those regarding the analysis of edible/useful plants and the structure of a nutrition survey, and has received and discussed numerous progress reports (often in person) from the researchers.
These five years have been times of high stress in the community. Often it seemed patently unfair that a community that was living among
Future Activities:
Journal Articles: 45 Displayed | Download in RIS Format
| Other center views: | All 63 publications | 46 publications in selected types | All 45 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Afeiche M, Peterson KE, Sanchez BN, Schnaas L, Cantonwine D, Ettinger AS, Solano-Gonzalez M, Hernandez-Avila M, Hu H, Tellez-Rojo MM. Windows of lead exposure sensitivity, attained height, and body mass index at 48 months. The Journal of Pediatrics 2012;160(6):1044-1049. |
R831725 (Final) R834800 (2012) R834800 (2013) |
Exit |
|
|
Arora M, Weuve J, Schwartz J, Wright RO. Association of environmental cadmium exposure with pediatric dental caries. Environmental Health Perspectives 2008;116(6):821-825. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C001 (2008) R831725C003 (2007) R831725C004 (2007) |
|
|
|
Brain JD, Heilig E, Donaghey TC, Knutson MD, Wessling-Resnick M, Molina RM. Effects of iron status on transpulmonary transport and tissue distribution of Mn and Fe. American Journal of Respiratory Cell and Molecular Biology 2006;34(3):330-337. |
R831725 (2005) R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2005) R831725C003 (2007) R831725C003 (2008) R831725C004 (2007) |
Exit Exit |
|
|
Cantonwine D, Meeker JD, Hu H, Sanchez BN, Lamadrid-Figueroa H, Mercado-Garcia A, Fortenberry GZ, Calafat AM, Tellez-Rojo MM. Bisphenol A exposure in Mexico City and risk of prematurity:a pilot nested case control study. Environmental Health 2010;9:62. |
R831725 (Final) R834800 (2011) R834800 (2012) |
Exit Exit Exit |
|
|
Chou K, Wright RO. Phthalates in food and medical devices. Journal of Medical Toxicology 2006;2(3):126-135. |
R831725 (Final) |
Exit |
|
|
Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M. Manganese and iron transport across pulmonary epithelium. American Journal of Physiology–Lung Cellular and Molecular Physiology 2006;290(6):L1247-L1259. |
R831725 (2005) R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2005) R831725C003 (2007) R831725C003 (2008) R831725C004 (2007) |
Exit Exit |
|
|
Heilig E, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. Pharmacokinetics of pulmonary manganese absorption: evidence for increased susceptibility to manganese loading in iron-deficient rats. American Journal of Physiology–Lung Cellular and Molecular Physiology 2005;288(5):L887-L893. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2005) R831725C003 (2007) R831725C004 (2007) |
Exit Exit |
|
|
Hopkins MR, Ettinger AS, Hernandez-Avila M, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Bellinger D, Hu H, Wright RO. Variants in iron metabolism genes predict higher blood lead levels in young children. Environmental Health Perspectives 2008;116(9):1261-1266. |
R831725 (2009) R831725 (Final) R831725C001 (2008) |
|
|
|
Hu H, Tellez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, Schwartz J, Schnaas L, Mercado-Garcia A, Hernandez-Avila M. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environmental Health Perspectives 2006;114(11):1730-1735. |
R831725 (2007) R831725 (2009) R831725 (Final) |
|
|
|
Hu H, Shine J, Wright RO. The challenge posed to children’s health by mixtures of toxic waste: the Tar Creek Superfund Site as a case-study. Pediatric Clinics of North America 2007;54(1):155-175. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2007) R831725C004 (2007) |
Exit |
|
|
Lanphear BP, Wright RO, Dietrich KN. Environmental neurotoxins. Pediatrics in Review 2005;26(6):191-198. |
R831725 (2009) R831725 (Final) R831725C001 (2005) R829389 (2003) R829389 (2004) R829389 (2005) R829389 (Final) |
Exit |
|
|
Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environmental Health Perspectives 2005;113(10):1376-1380. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2005) |
|
|
|
Ortega Garcia JA, Carrizo Gallardo D, Ferris i Tortajada J, Garcia MM, Grimalt JO. Meconium and neurotoxicants: searching for a prenatal exposure timing. Archives of Disease in Childhood 2006;91(8):642-646. |
R831725 (2009) |
Exit |
|
|
Ostrea Jr EM, Morales V, Ngoumgna E, Prescilla R, Tan E, Hernandez E, Ramirez GB, Cifra HL, Manlapaz ML. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. NeuroToxicology 2002;23(3):329-339. |
R831725 (2009) |
Exit Exit |
|
|
Schaider LA, Senn DB, Brabander DJ, McCarthy KD, Shine JP. Characterization of zinc, lead, and cadmium in mine waste: implications for transport, exposure, and bioavailability. Environmental Science and Technology 2007;41(11):4164-4171. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2007) R831725C004 (2007) |
Exit |
|
|
Surkan PJ, Schnaas L, Wright RJ, Tellez-Rojo MM, Lamadrid-Figueroa H, Hu H, Hernandez-Avila EM, Bellinger DC, Schwartz J, Perroni E, Wright RO. Maternal self-esteem, exposure to lead, and child neurodevelopment. NeuroToxicology 2008;29(2):278-285. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C001 (2008) R831725C003 (2007) R831725C004 (2007) |
Exit Exit |
|
|
Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-Garcia A, Schnaas-Arrieta L, Wright RO, Hernandez-Avila M, Hu H. Longitudinal associations between blood lead concentrations lower than 10 μg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics 2006;118(2):e323-e330. |
R831725 (2007) R831725 (2009) R831725 (Final) R835441 (2017) |
Exit |
|
|
Thompson K, Molina RM, Brain JD, Wessling-Resnick M. Belgrade rats display liver iron loading. Journal of Nutrition 2006;136(12):3010-3014. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2007) R831725C003 (2008) R831725C004 (2007) |
Exit Exit |
|
|
Thompson K, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. The influence of high iron diet on rat lung manganese absorption. Toxicology and Applied Pharmacology 2006;210(1-2):17-23. |
R831725 (2005) R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2005) R831725C003 (2007) R831725C003 (2008) R831725C004 (2007) |
Exit Exit |
|
|
Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB Journal 2007;21(1):223-230. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2007) R831725C003 (2008) R831725C004 (2007) |
Exit |
|
|
Thompson K, Molina RM, Donaghey T, Brain JD, Wessling-Resnick M. Iron absorption by Belgrade rat pups during lactation. American Journal of Physiology-Gastrointestinal and Liver Physiology 2007;293(3):G640-G644. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2007) R831725C003 (2008) R831725C004 (2007) |
Exit Exit |
|
|
Turker G, Ergen K, Karakoc Y, Arisoy AE, Barutcu UB. Concentrations of toxic metals and trace elements in the meconium of newborns from an industrial city. Biology of the Neonate 2006;89(4):244-250. |
R831725 (2009) |
Exit |
|
|
Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. NeuroToxicology 2006;27(2):210-216. |
R831725 (2005) R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2005) |
Exit Exit |
|
|
Wright RO, Baccarelli A. Metals and neurotoxicology. The Journal of Nutrition 2007;137(12):2809-2813. |
R831725 (2007) R831725 (2009) R831725 (Final) R831725C001 (2007) R831725C003 (2007) R831725C004 (2007) |
Exit Exit |
|
|
Wright RO, Fields N. Therapeutics and toxicology. Current Opinion in Pediatrics 2008;20(2):171. |
R831725 (2009) R831725 (Final) R831725C001 (2008) |
Exit Exit |
|
|
Wright RO. Neurotoxicology: what can context teach us? Journal of Pediatrics 2008;152(2):155-157. |
R831725 (2009) R831725 (Final) R831725C001 (2008) |
Exit Exit |
|
|
Wright RO. New morbidities: new challenges. Current Opinion in Pediatrics 2009;21(2):220-221. |
R831725 (2009) R831725 (Final) R831725C001 (2008) |
Exit Exit |
|
|
Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, Wright RO. Maternal blood manganese levels and infant birth weight. Epidemiology 2009;20(3):367-373. |
R831725 (2009) R831725 (Final) R831725C001 (2008) |
Exit |
|
|
Collins JF, Wessling-Resnick M, Knutson MD. Hepcidin regulation of iron transport. The Journal of Nutrition 2008;138(11):2284-2288. |
R831725 (Final) |
|
|
|
Zota AR, Willis R, Jim R, Norris GA, Shine JP, Duvall RM, Schaider LA, Spengler JD. Impact of mine waste on airborne respirable particulates in northeastern Oklahoma, United States. Journal of the Air & Waste Management Association 2009;59(11):1347-1357. |
R831725 (Final) |
Exit |
|
|
Pilsner R, Hu H, Ettinger A, Sanchez B, Wright R, Cantonwine D, Lazarus A, Lamadrid-Figueroa H, Mercado-García A, Téllez-Rojo MM, Hernández-Avila M. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Epidemiology 2009;20(6):S84. |
R831725 (Final) |
|
|
|
Ettinger AS, Zota AR, Amarasiriwardena CJ, Hopkins MR, Schwartz J, Hu H, Wright RO. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environmental Health Perspectives 2009;117(7):1059-1064. |
R831725 (Final) |
|
|
|
Deb S, Johnson EE, Robalinho-Teixeira RL, Wessling-Resnick M. Modulation of intracellular iron levels by oxidative stress implicates a novel role for iron in signal transduction. Biometals 2009;22:855-862. |
R831725 (Final) |
|
|
|
Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environmental Health Perspectives 201;119(3):409-415. |
R831725 (Final) |
|
|
|
Cantonwine D, Hu H, Sánchez BN, Lamadrid-Figueroa H, Smith D, Ettinger AS, Mercado-García A, Hernández-Avila M, Wright RO, Téllez-Rojo MM. Critical windows of fetal lead exposure:adverse impacts on length of gestation and risk of premature delivery. Journal of Occupational and Environmental Medicine 2010;52(11):1106-1111. |
R831725 (Final) |
|
|
|
Cantonwine D, Hu H, Téllez-Rojo MM, Sánchez BN, Lamadrid-Figueroa H, Ettinger AS, Mercado-García A, Hernández-Avila M, Wright RO. HFE gene variants modify the association between maternal lead burden and infant birthweight:a prospective birth cohort study in Mexico City, Mexico. Environmental Health 2010;9:1-9. |
R831725 (Final) |
|
|
|
Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annual Review of Nutrition 2010;30(1):105-122. |
R831725 (Final) |
|
|
|
Pilsner JR, Hu H, Wright RO, Kordas K, Ettinger AS, Sanchez BN, Cantonwine D, Lazarus AL, Cantoral A, Schnaas L, Tellez-Rojo MM. Maternal MTHFR genotype and haplotype predict deficits in early cognitive development in a lead-exposed birth cohort in Mexico City. The American Journal of Clinical Nutrition 2010;92(1):226-234. |
R831725 (Final) |
|
|
|
Molina RM, Phattanarudee S, Kim J, Thompson K, Wessling-Resnick M, Maher TJ, Brain JD. Ingestion of Mn and Pb by rats during and after pregnancy alters iron metabolism and behavior in offspring. Neurotoxicology 2011;32(4):413-422. |
R831725 (Final) |
|
|
|
Thompson KJ, Molina RM, Donaghey T, Savaliya S, Schwob JE, Brain JD. Manganese uptake and distribution in the brain after methyl bromide-induced lesions in the olfactory epithelia. Toxicological Sciences 2011;120(1):163-172. |
R831725 (Final) |
|
|
|
Zota AR, Schaider LA, Ettinger AS, Wright RO, Shine JP, Spengler JD. Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. Journal of Exposure Science & Environmental Epidemiology 2011;21(5):495-505. |
R831725 (Final) |
not available |
|
|
Adamkiewicz G, Zota AR, Fabian MP, Chahine T, Julien R, Spengler JD, Levy JI. Moving environmental justice indoors:understanding structural influences on residential exposure patterns in low-income communities. American Journal of Public Health 2011;101(S1):S238-245. |
R831725 (Final) |
|
|
|
Afeiche M, Peterson KE, Sánchez BN, Cantonwine D, Lamadrid-Figueroa H, Schnaas L, Ettinger AS, Hernández-Avila M, Hu H, Téllez-Rojo MM. Prenatal lead exposure and weight of 0-to 5-year-old children in Mexico City. Environmental Health Perspectives 2011;119(10):1436-1441. |
R831725 (Final) |
not available |
|
|
Sánchez BN, Kang S, Mukherjee B. A latent variable approach to study gene–environment interactions in the presence of multiple correlated exposures. Biometrics 2012;68(2):466-476. |
R831725 (Final) |
|
|
|
Zhang A, Hu H, Sánchez BN, Ettinger AS, Park SK, Cantonwine D, Schnaas L, Wright RO, Lamadrid-Figueroa H, Tellez-Rojo MM. Association between prenatal lead exposure and blood pressure in children. Environmental Health Perspectives 2012;120(3):445-450. |
R831725 (Final) |
|
Supplemental Keywords:
children, Native American, tribal, mixtures, lead, PBPK, community, Superfund, intervention, , environmental management, Scientific Discipline, Health, RFA, Arsenic, Risk Assessment, Health Risk Assessment, Epidemiology, Immunology, Children's Health, Biochemistry, Environmental Chemistry, neurodevelopmental toxicity, developmental toxicity, children's environmental health, mining waste, community-based intervention, biological markers, metals;, Health, RFA, Scientific Discipline, ENVIRONMENTAL MANAGEMENT, Health Risk Assessment, Immunology, Environmental Chemistry, Epidemiology, Arsenic, Children's Health, Biochemistry, Risk Assessment, Human Health Risk Assessment, biological markers, developmental toxicity, neurodevelopmental toxicity, mining waste, community-based intervention, metalsProgress and Final Reports:
Original Abstract Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R831725C001 Metals, Nutrition, and Stress in Child Development
R831725C002 Exposure Assessment of Children and Metals in Mining Waste: Composition, Environmental Transport, and Exposure Patterns
R831725C003 Manganese, Iron, Cadmium, and Lead Transport from the Environment to Critical Organs During Gestation and Early Development in a Rat Model
R831725C004 Metals Neurotoxicity Research Project
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2008
- 2007 Progress Report
- 2006
- 2005 Progress Report
- 2004 Progress Report
- Original Abstract
45 journal articles for this center
