Grantee Research Project Results
2008 Progress Report: Characterization and Source Apportionment
EPA Grant Number: R832415C001Subproject: this is subproject number 001 , established and managed by the Center Director under grant R832415
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Rochester PM Center
Center Director: Oberdörster, Günter
Title: Characterization and Source Apportionment
Investigators: Hopke, Philip K. , Prather, Kimberly A.
Current Investigators: Hopke, Philip K. , Prather, Kimberly A. , Gelein, Robert
Institution: Clarkson University , University of Rochester , University of California - San Diego
Current Institution: Clarkson University , University of California - San Diego , University of Rochester
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
Project Period Covered by this Report: October 1, 2007 through September 30, 2008
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
Core 1: Exposure Assessment and PM Source Identification
Introduction
A central hypothetical mechanism of how particles affect human health involves the generation of reactive oxygen species (ROS) at target sites in the lung. ROS has been defined to include families of oxygen-centered or related free radicals, ions, and molecules. The free radical family includes hydroxyl, hydroperoxyl, and organic peroxy radicals. Ions such as the superoxide, hypochlorite, and peroxynitrite ions, and molecules such as hydrogen peroxide, organic and inorganic peroxides also come under the umbrella of ‘Reactive Oxygen Species'. Much of the attention has focused on the formation of ROS in situ after particle deposition in the respiratory tract generally through the interaction with transition metal ions (Stohs et al. 1997), organic hydrocarbons, such as polycyclic aromatic hydrocarbons and quinones (Squadrito et al. 2001), and ultrafine particle surfaces (Li et al. 2003). However, recent work has shown that ROS is present in the atmosphere on respirable particles to which we are exposed (Hung and Wang 2001, Hasson and Paulson 2003, Venkatachari et al. 2005, 2007). The hypothesis that the ROS present on particles could cause the same kind of systemic dysfunction as endogenously generated ROS has clear merit and represents a fundamental issue for further investigation. Thus, a major function of this core is the study of the concentrations of particle-bound ROS in ambient PM2.5 and studies to better understand their formation so that we can eventually estimate the amounts of particle bound ROS arising from anthropogenic and biogenic sources of the reactive hydrocarbon precursor species.
Progress Summary:
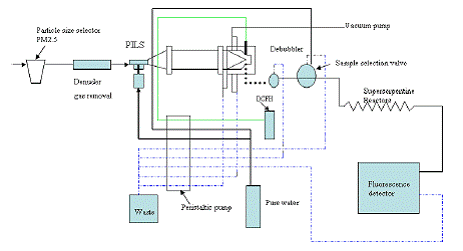
Journal Articles on this Report : 2 Displayed | Download in RIS Format
| Other subproject views: | All 19 publications | 13 publications in selected types | All 13 journal articles |
|---|---|---|---|
| Other center views: | All 191 publications | 157 publications in selected types | All 144 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Venkatachari P, Hopke PK. Development and laboratory testing of an automated monitor for the measurement of atmospheric particle-bound reactive oxygen species (ROS). Aerosol Science and Technology 2008;42(8):629-635. |
R832415 (2007) R832415 (2008) R832415 (2010) R832415 (2011) R832415 (Final) R832415C001 (2008) R832415C001 (2010) R832415C001 (2011) |
Exit Exit Exit |
|
|
Yue W, Stolzel M, Cyrys J, Pitz M, Heinrich J, Kreyling WG, Wichmann H-E, Peters A, Wang S, Hopke PK. Source apportionment of ambient fine particle size distribution using positive matrix factorization in Erfurt, Germany. Science of the Total Environment 2008;398(1-3):133-144. |
R832415 (2007) R832415 (2008) R832415 (2010) R832415 (2011) R832415 (Final) R832415C001 (2008) R832415C001 (2010) R832415C001 (2011) R832415C002 (2006) R832415C002 (2008) R832415C002 (2010) R832415C002 (2011) R827354 (Final) R834797 (2016) |
Exit Exit Exit |
Supplemental Keywords:
RFA, Air, Scientific Discipline, Health Risk Assessment, particulate matter, Environmental Chemistry, Biochemistry, chemical characteristics, atmospheric chemistry, fine particles, human exposure, airborne particulate matter, aerosol composition, cardiopulmonary responses, atmospheric particlesProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832415 Rochester PM Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832415C001 Characterization and Source Apportionment
R832415C002 Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
R832415C003 Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
R832415C004 Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
R832415C005 Ultrafine Particle Cell Interactions In Vitro: Molecular Mechanisms Leading To Altered Gene Expression in Relation to Particle Composition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2011 Progress Report
- 2010 Progress Report
- 2009 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
13 journal articles for this subproject
Main Center: R832415
191 publications for this center
144 journal articles for this center
