Grantee Research Project Results
2011 Progress Report: Environmental Transformation And Biological Fate Of Fresh And Aged Cerium Oxide Nanoparticles
EPA Grant Number: R834860Title: Environmental Transformation And Biological Fate Of Fresh And Aged Cerium Oxide Nanoparticles
Investigators: Jolliet, Olivier , Harkema, Jack , Wagner, James , Morishita, Masako , Wooldridge, Margaret , Emond, Claude
Current Investigators: Jolliet, Olivier , Keeler, Gerald J. , Emond, Claude , Harkema, Jack , Wagner, James , Wooldridge, Margaret , Morishita, Masako
Institution: University of Michigan , Michigan State University , University of Montreal
Current Institution: University of Michigan , Michigan State University
EPA Project Officer: Aja, Hayley
Project Period: March 1, 2011 through June 30, 2013 (Extended to February 28, 2016)
Project Period Covered by this Report: March 1, 2011 through February 29,2012
Project Amount: $600,000
RFA: Increasing Scientific Data on the Fate, Transport and Behavior of Engineered Nanomaterials in Selected Environmental and Biological Matrices (2010) RFA Text | Recipients Lists
Research Category: Chemical Safety for Sustainability
Objective:
Progress Summary:
 Figure 1. Powder samples of seria particles produced by using the University of Michigan combustion synthesis facility. |  |
1b) Atmospheric transformation of CeO2 NPs: In collaboration with Ingeniven Inc. (North Hampton, NH), we designed an atmospheric reaction chamber (a fluorinated ethylene propylene Teflon bag) and its enclosure during this reporting period (shown in Figure 2). After a comprehensive search for lamps that can simulate the UV portion of the solar spectrum and fixtures with an acceptable price, we found UV-340 lamps to be an excellent choice. Our chamber will have about 250 W/m2 of direct UV flux in the chamber, which is equivalent to five times solar UV flux. Reflections from the interior surface of the enclosure are expected to significantly increase this figure; actual UV flux levels will be measured with a UV meter when the chamber is completed.
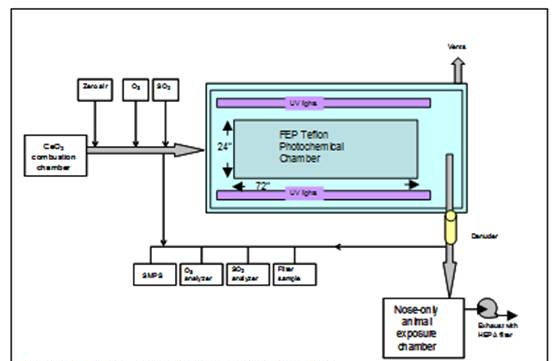
Figure 2. Schematic of the reaction chamber.
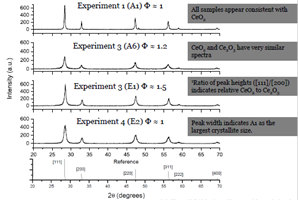 Figure 3. XRD spectra of ceria samples 1-4 corresponding to the samples presented in Figure 1 and Tables 1 and 2. |
|
 | 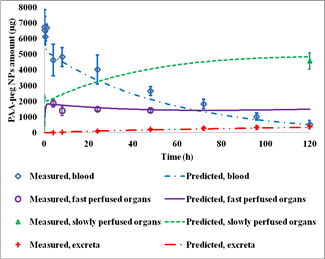 |
| Figure 4. General framework of the PBTK model for i.v. injection of NPs. | Figure 5. Measured v.s. PBTK predicted of PAA-peg NPs following i.v. injection in rats |
- The partition coefficient between tissue: blood and macrophage uptake capacity per tissue weight determine the maximum capacity in tissues.
- The maximum uptake rate of macrophages determines the increase rate of PAA-peg NPs captured by macrophages.
- The transfer factor from capillary blood to tissues determines the rate of the distribution of NPs in all compartments.
Future Activities:
Journal Articles:
No journal articles submitted with this report: View all 25 publications for this projectSupplemental Keywords:
toxicokinetics, pharmacokinetic, nanotoxicology, engineered nanomaterials, cerium oxide, CeO2, inhalation exposure, in vivo, in vitroProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
