Grantee Research Project Results
2013 Progress Report: Consortium for Manufactured Nanomaterial Bioavailability & Environmental Exposure
EPA Grant Number: R834575Title: Consortium for Manufactured Nanomaterial Bioavailability & Environmental Exposure
Investigators: Colvin, Vicki L. , Klaine, Stephen J. , Tyler, Charles , Valsami-Jones, Eugenia , Lead, Jamie , Chipman, Kevin , Viant, Mark , Luoma, Sam , Fernandes, Teresa , Stone, Vicki
Current Investigators: Lead, Jamie , Alvarez, Pedro J. , Colvin, Vicki L. , Klaine, Stephen J. , Luoma, Sam , Stone, Vicki , Tyler, Charles , Valsami-Jones, Eugenia , Chipman, Kevin , Viant, Mark , Fernandes, Teresa
Institution: Rice University , University of California - Davis , Natural History Museum (London) , University of Birmingham , Edinburgh Napier University , Clemson University , University of Exeter
Current Institution: Rice University , Clemson University , University of California - Davis , University of Exeter , University of Birmingham , Edinburgh Napier University , Natural History Museum (London)
EPA Project Officer: Aja, Hayley
Project Period: August 1, 2010 through August 1, 2013 (Extended to December 31, 2015)
Project Period Covered by this Report: August 1, 2012 through July 31,2013
Project Amount: $2,000,000
RFA: Environmental Behavior, Bioavailability and Effects of Manufactured Nanomaterials - Joint US – UK Research Program (2009) RFA Text | Recipients Lists
Research Category: Chemical Safety for Sustainability
Objective:
In order to fully and sustainably apply nanotechnology innovations, it is crucial to minimize any unintended environmental impacts resulting from the application of manufactured nanomaterials (NM). To realize this vision, industry and policymakers must base risk management decisions on sound scientific information about the environmental fate of NM; their availability to receptor organisms (including related concepts such as uptake); and any resultant biological effects (toxicity). This basic knowledge can be effectively conveyed by validated models that describe NM behavior and relate these data to information about NM structure and relevant environmental chemistry. These models, whether conceptual or predictive in nature, will give decisionmakers the tools to grapple with the nearly infinite forms of possible NM, as well as explore the effects of various risk mitigation strategies. Towards this end, the nanoBEE consortium will develop and refine, using empirical data, a critical subset of models focused on exposure to NM and their bioavailability in the environment.
The methodological approach in the nanoBEE consortium is summarized in Figure 1. Tunable and uniform NM’s are characterized appropriately with existing and novel methods developed in this consortium. The libraries will underpin steady-state and dynamic laboratory and mesocosm experiments to understand NM environmental chemistry, transport, bioavailability and toxicity. These data sets are being used to validate exposure, bioavailability and toxicity based models, and then merged and refined through experiments. The result will be an integrated and validated set of models (EEM-BDM-BLM) (EEM: Environmental Exposure Model; BDM: Biodynamic Model; BLM: Biotic Ligand Model), with, we anticipate, some direct integration between BDM-BLM. Research in the nanoBEE consortium is structured by 5 primary objectives (listed below), and 12 tasks. The produced data and models will link to risk assessment modelling strategies.
Aim 1: Nanomaterial library production, characterization
Aim 2: Aqueous electron tomography of nanomaterials
Aim 3: Environmental chemistry, fate and exposure models (EEMs)
Aim 4: NM association with organisms and the biodynamic model
Aim 5: Effects on aquatic organisms – towards a nano-BLM model
Progress Summary:
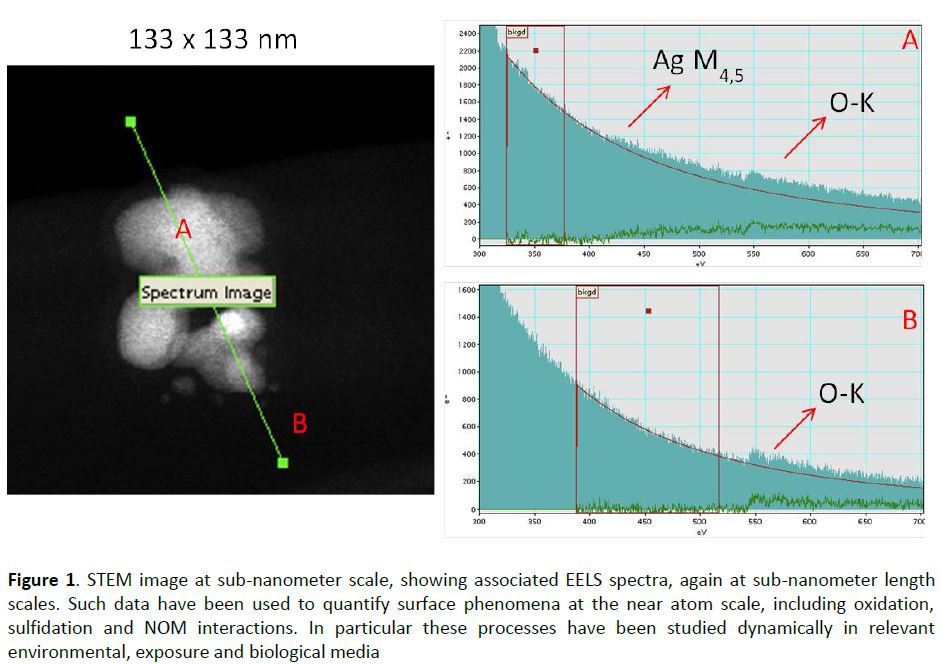
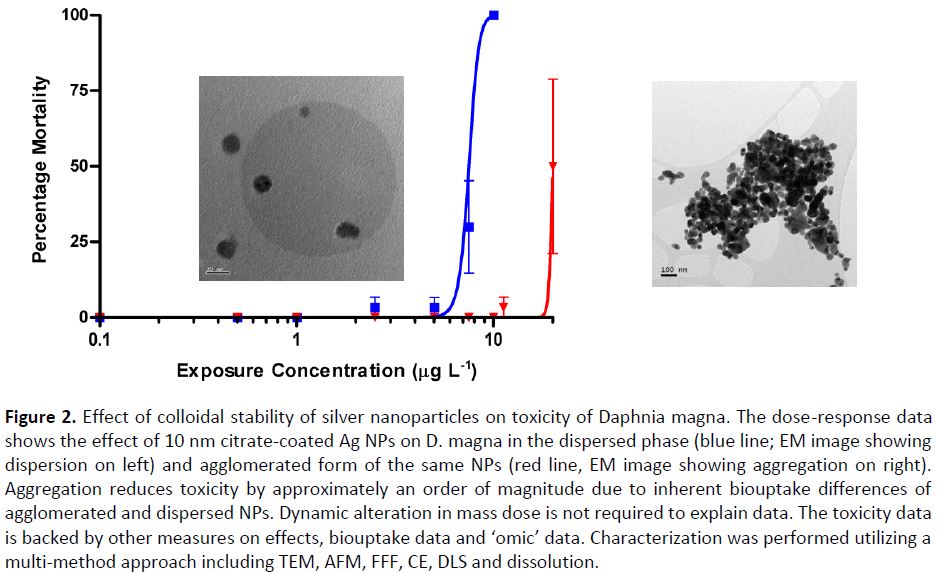
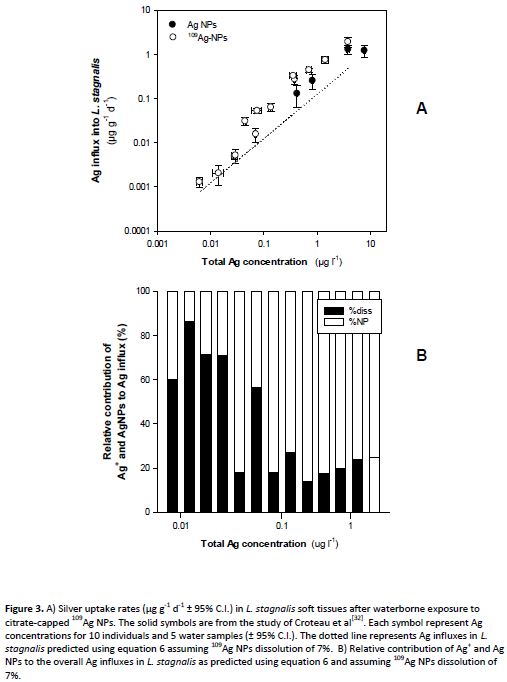
Finally, the various exposures performed on a range of organisms have partly been modelled, especially using the biodynamic model and further work is ongoing with the biotic ligand model. In a number of cases we were able to perform modelling on exposures to the same organisms, improving data utility. Figure 3 shows that at low exposure concentrations (<0.1 µg/l), 70% of the bioaccumulated Ag can be ascribed to uptake from newly solubilized Ag, whereas 80% of the bioaccumulated Ag originated from the AgNPs above this concentration, i.e., there are concentration-dependent changes in the species or form of the silver that is bioaccumulated.
Journal Articles on this Report : 20 Displayed | Download in RIS Format
| Other project views: | All 37 publications | 37 publications in selected types | All 35 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Contreras EQ, Cho M, Zhu H, Puppala HL, Escalera G, Zhong W, Colvin VL. Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environmental Science & Technology 2013;47(2):1148-1154. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Croteau M-N, Misra SK, Luoma SN, Valsami-Jones E. Silver bioaccumulation dynamics in a freshwater invertebrate after aqueous and dietary exposures to nanosized and ionic Ag. Environmental Science & Technology 2011;45(15):6600-6607. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Croteau M-N, Cain DJ, Fuller CC. Novel and nontraditional use of stable isotope tracers to study metal bioavailability from natural particles. Environmental Science & Technology 2013;47(7):3424-3431. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Glenn JB, White SA, Klaine SJ. Interactions of gold nanoparticles with freshwater aquatic macrophytes are size and species dependent. Environmental Toxicology and Chemistry 2012;31(1):194-201. |
R834575 (2013) R834575 (Final) R834092 (Final) |
Exit Exit |
|
|
Glenn JB, Klaine SJ. Abiotic and biotic factors that influence the bioavailability of gold nanoparticles to aquatic macrophytes. Environmental Science & Technology 2013;47(18):10223-10230. |
R834575 (2013) R834575 (Final) R834092 (Final) |
Exit Exit Exit |
|
|
Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of nanomaterials in the environment. Environmental Science & Technology 2012;46(13):6893-6899. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Merrifield RC, Wang ZW, Palmer RE, Lead JR. Synthesis and characterization of polyvinylpyrrolidone coated cerium oxide nanoparticles. Environmental Science & Technology 2013;47(21):12426-12433. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Misra SK, Dybowska A, Berhanu D, Luoma SN, Valsami-Jones E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. The Science of the Total Environment 2012;438:225-232. |
R834575 (2013) R834575 (Final) R834557 (Final) |
Exit Exit Exit |
|
|
Newton KM, Puppala HL, Kitchens CL, Colvin VL, Klaine SJ. Silver nanoparticle toxicity to Daphnia magna is a function of dissolved silver concentration. Environmental Toxicology and Chemistry 2013;32(10):2356-2364. |
R834575 (2013) R834575 (Final) |
Exit Exit |
|
|
Osborne OJ, Johnston BD, Moger J, Balousha M, Lead JR, Kudoh T, Tyler CR. Effects of particle size and coating on nanoscale Ag and TiO2 exposure in zebrafish (Danio rerio) embryos. Nanotoxicology 2013;7(8):1315-1324. |
R834575 (2013) R834575 (Final) |
Exit Exit |
|
|
Romer I, Gavin AJ, White TA, Merrifield RC, Chipman JK, Viant MR, Lead JR. The critical importance of defined media conditions in Daphnia magna nanotoxicity studies. Toxicology Letters 2013;223(1):103-108. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Seda BC, Ke P-C, Mount AS, Klaine SJ. Toxicity of aqueous C70-gallic acid suspension in Daphnia magna. Environmental Toxicology and Chemistry 2012;31(1):215-220. |
R834575 (2013) R834575 (Final) R834092 (Final) |
Exit Exit |
|
|
Tejamaya M, Romer I, Merrifield RC, Lead JR. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environmental Science & Technology 2012;46(13):7011-7017. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
van Aerle R, Lange A, Moorhouse A, Paszkiewicz K, Ball K, Johnston BD, de-Bastos E, Booth T, Tyler CR, Santos EM. Molecular mechanisms of toxicity of silver nanoparticles in zebrafish embryos. Environmental Science & Technology 2013;47(14):8005-8014. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Xiu Z-M, Ma J, Alvarez PJJ. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environmental Science & Technology 2011;45(20):9003-9008. |
R834575 (2011) R834575 (2013) R834575 (Final) |
Exit Exit Exit |
|
|
Xiu Z-M, Zhang Q-B, Puppala HL, Colvin VL, Alvarez PJJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Letters 2012;12(8):4271-4275. |
R834575 (2013) R834575 (Final) R834557 (Final) |
Exit Exit Exit |
|
|
Yang Y, Mathieu JM, Chattopadhyay S, Miller JT, Wu T, Shibata T, Guo W, Alvarez PJJ. Defense mechanisms of Pseudomonas aeruginosa PAO1 against quantum dots and their released heavy metals. ACS Nano 2012;6(7):6091-6098. |
R834575 (2013) R834575 (Final) R834557 (Final) |
Exit Exit Exit |
|
|
Yang Y, Wang J, Zhu H, Colvin VL, Alvarez PJJ. Relative susceptibility and transcriptional response of nitrogen cycling bacteria to quantum dots. Environmental Science & Technology 2012;46(6):3433-3441. |
R834575 (2013) R834575 (Final) R834557 (Final) |
Exit Exit Exit |
|
|
Yang Y, Wang J, Xiu Z, Alvarez PJJ. Impacts of silver nanoparticles on cellular and transcriptional activity of nitrogen-cycling bacteria. Environmental Toxicology and Chemistry 2013;32(7):1488-1494. |
R834575 (2013) R834575 (Final) |
Exit |
|
|
Yang Y, Quensen J, Mathieu J, Wang Q, Wang J, Li M, Tiedje JM, Alvarez PJJ. Pyrosequencing reveals higher impact of silver nanoparticles than Ag+ on the microbial community structure of activated sludge. Water Research 2014;48:317-325. |
R834575 (2013) R834575 (Final) |
Exit Exit Exit |
Progress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.