Grantee Research Project Results
2008 Progress Report: Metals Neurotoxicity Research Project
EPA Grant Number: R831725C004Subproject: this is subproject number 004 , established and managed by the Center Director under grant R831725
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Health Effects Institute (2015 - 2020)
Center Director: Greenbaum, Daniel S.
Title: Metals Neurotoxicity Research Project
Investigators: Maher, Tim , Weisskopf, Marc
Institution: Harvard University
EPA Project Officer: Callan, Richard
Project Period: June 1, 2004 through May 31, 2009 (Extended to May 31, 2011)
Project Period Covered by this Report: June 1, 2008 through May 31,2009
RFA: Centers for Children's Environmental Health and Disease Prevention Research (2003) RFA Text | Recipients Lists
Research Category: Human Health , Children's Health
Objective:
In project 4 (R831725C004), we focused on the long term effect of Perinatal exposure to different concentrations of manganese (as MnCl2) and lead (as Pb acetate) individually on neurochemistry and behavior in male and female Sprague- Dawley rats. During the past year, we completed the three different concentrations of MnCl2 (1.25, 5 and 10 mg/ml), and for Pb acetate we have completed a wide range of doses in order to especially obtain blood Pb levels lower than 10 µg/dL.
Progress Summary:
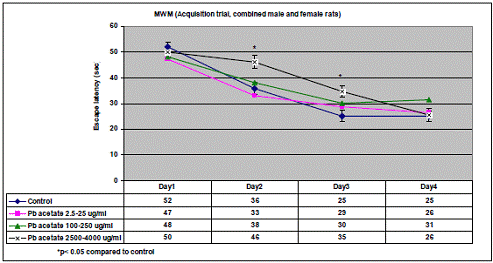
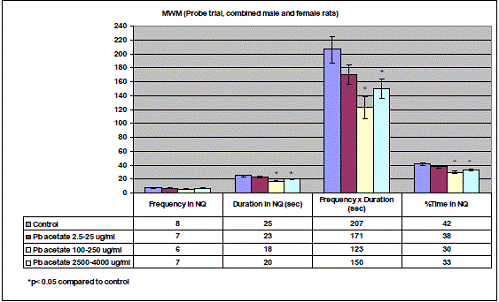
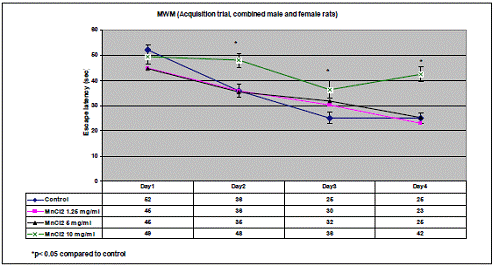
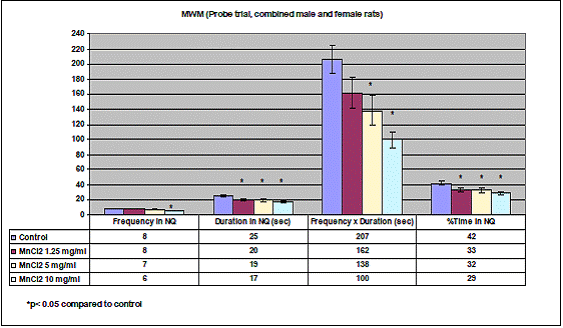
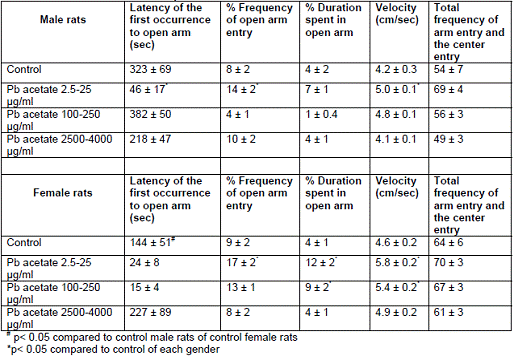

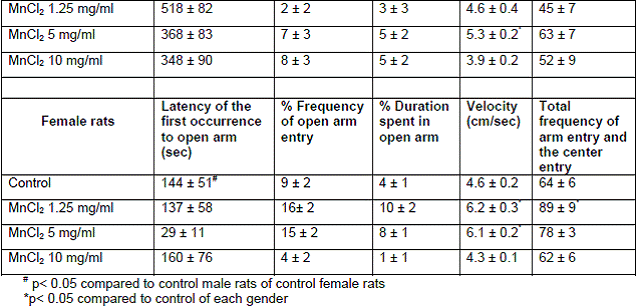
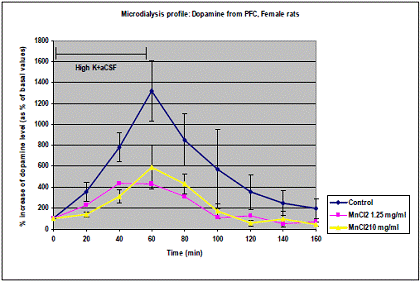
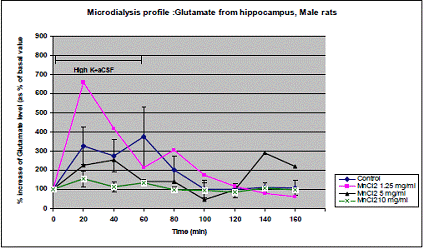
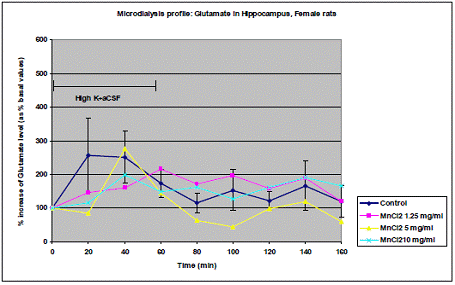
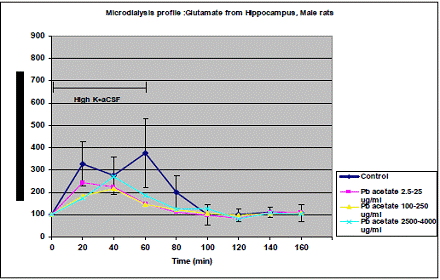
Journal Articles:
No journal articles submitted with this report: View all 12 publications for this subprojectSupplemental Keywords:
children, Native American, tribal, mixtures, lead, PBPK, community, Superfund, intervention, environmental management, environmental management, international cooperation, Scientific Discipline, Waste, Health, RFA, Risk Assessment, Health Risk Assessment, Children's Health, Hazardous Waste, Biochemistry, Environmental Chemistry, Hazardous, neurodevelopmental toxicity, developmental toxicity, fate and transport , children's environmental health, mining wastes, human health risk, mining waste, community-based intervention, metal contamination, metal wastes, metals,, RFA, Health, Scientific Discipline, INTERNATIONAL COOPERATION, ENVIRONMENTAL MANAGEMENT, Waste, Environmental Chemistry, Health Risk Assessment, Hazardous Waste, Biochemistry, Children's Health, Hazardous, Risk Assessment, community-based intervention, fate and transport , developmental toxicity, Human Health Risk Assessment, neurodevelopmental toxicity, children's environmental health, mining waste, metal wastes, metals, human health risk, metal contaminationProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R831725 Health Effects Institute (2015 - 2020) Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R831725C001 Metals, Nutrition, and Stress in Child Development
R831725C002 Exposure Assessment of Children and Metals in Mining Waste: Composition, Environmental Transport, and Exposure Patterns
R831725C003 Manganese, Iron, Cadmium, and Lead Transport from the Environment to Critical Organs During Gestation and Early Development in a Rat Model
R831725C004 Metals Neurotoxicity Research Project
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
12 journal articles for this subproject
Main Center: R831725
35 publications for this center
25 journal articles for this center
