Grantee Research Project Results
Final Report: Project 4 -- Transport and Fate Particles
EPA Grant Number: R832414C004Subproject: this is subproject number 004 , established and managed by the Center Director under grant R832414
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: UC Davis Center for Children's Environmental Health and Disease Prevention
Center Director: Van de Water, Judith
Title: Project 4 -- Transport and Fate Particles
Investigators: Wilson, Dennis , Louie, Angelique
Institution: University of California - Davis
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2011)
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
Summary/Accomplishments (Outputs/Outcomes):
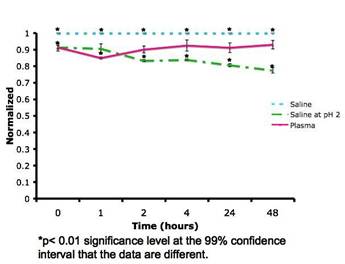
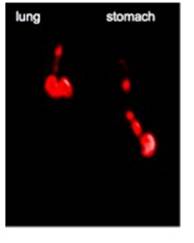
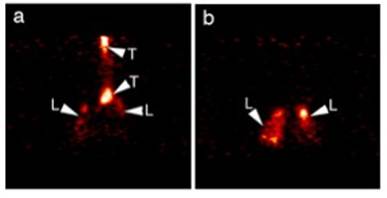
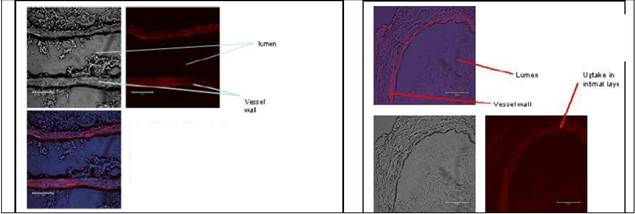
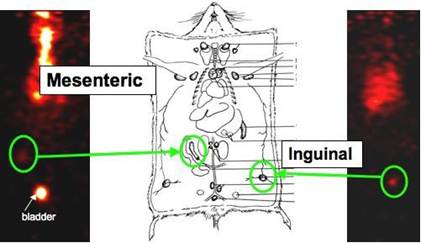
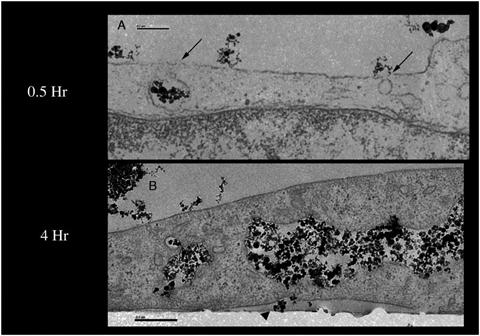
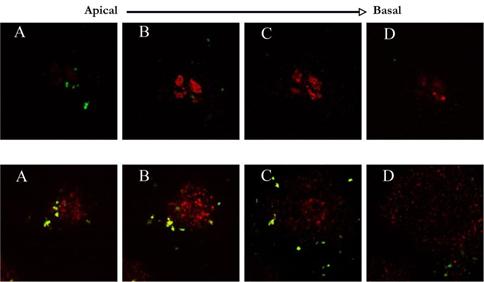
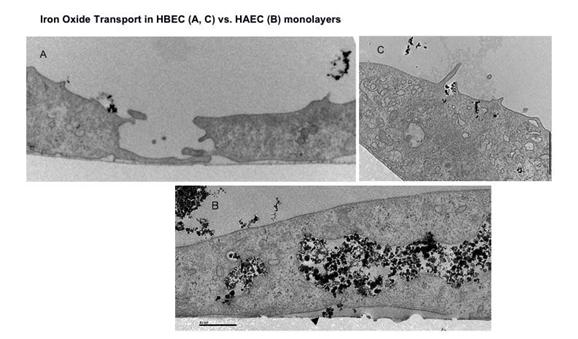
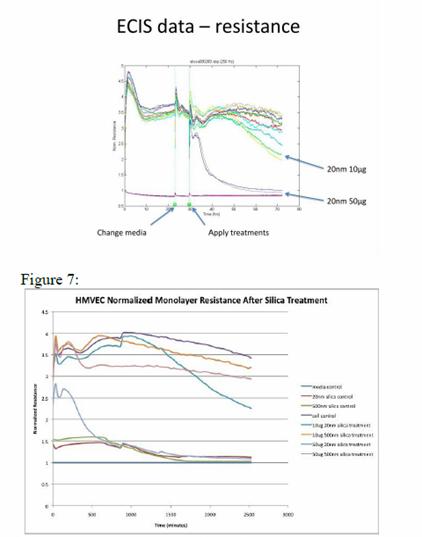
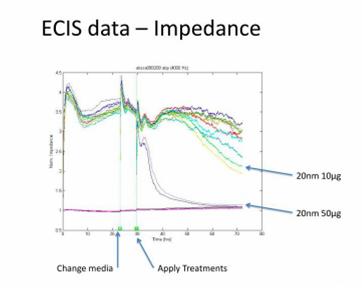
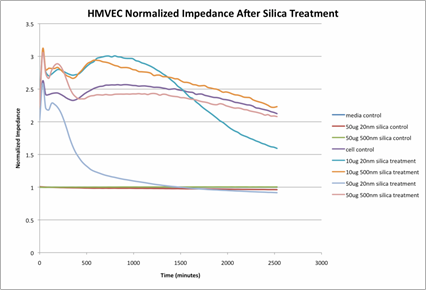
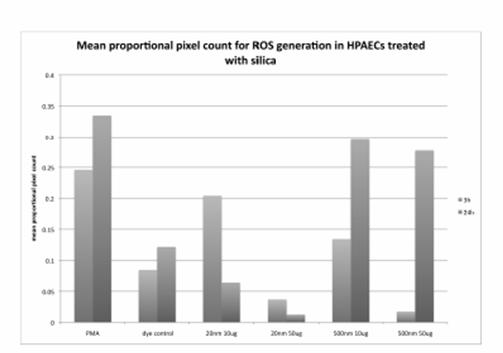
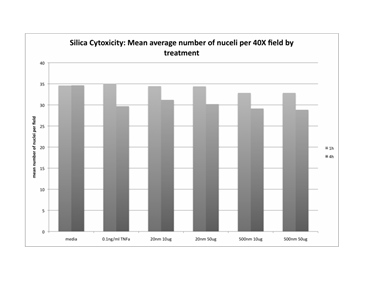
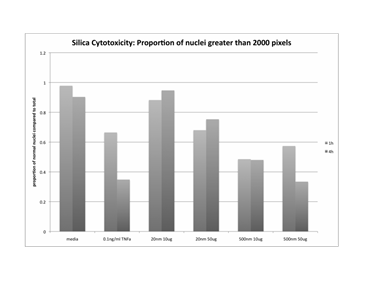
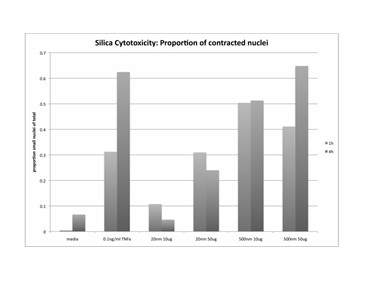
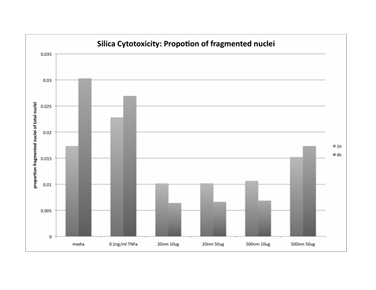
Journal Articles on this Report : 2 Displayed | Download in RIS Format
| Other subproject views: | All 3 publications | 2 publications in selected types | All 2 journal articles |
|---|---|---|---|
| Other center views: | All 128 publications | 71 publications in selected types | All 64 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
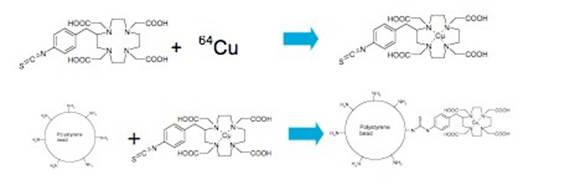
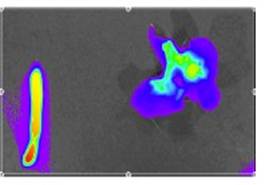
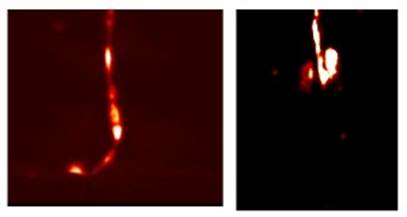
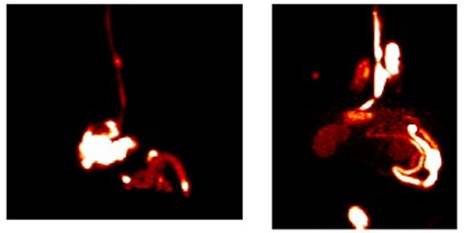
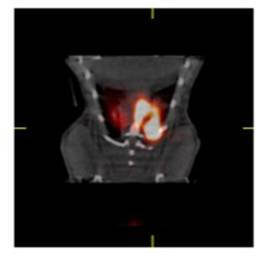
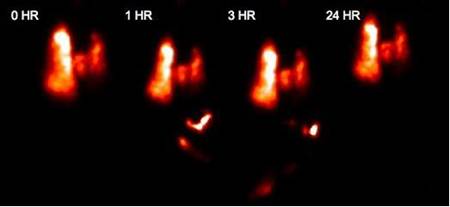
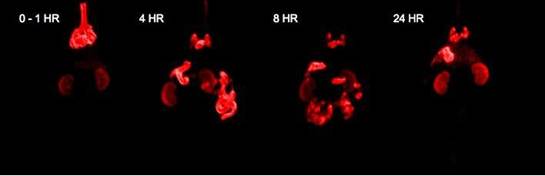
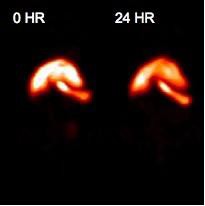
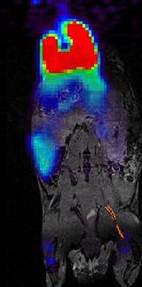
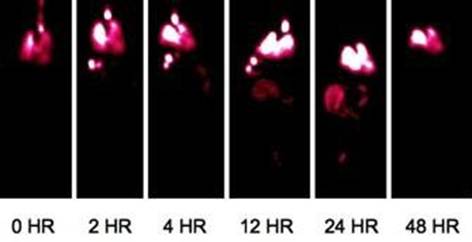
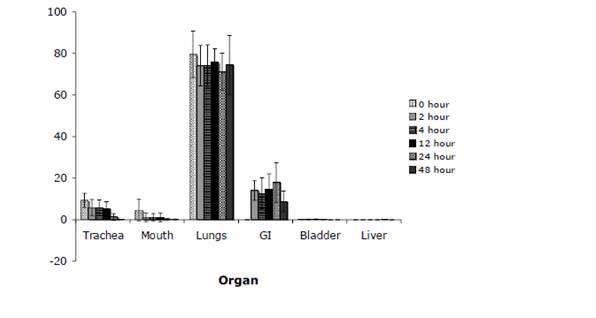
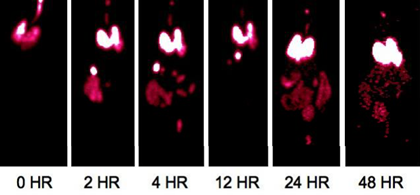
Enright HA, Bratt JM, Bluhm AP, Kenyon NJ, Louie AY. Tracking retention and transport of ultrafine polystyrene in an asthmatic mouse model using positron emission tomography. Experimental Lung Research 2013;39(7):304-313. |
R832414 (Final) R832414C004 (Final) |
Exit |
|
|
Palko HA, Fung JY, Louie AY. Positron emission tomography:a novel technique for investigating the biodistribution and transport of nanoparticles. Inhalation Toxicology 2010;22(8):657-688. |
R832414 (Final) R832414C004 (Final) |
Exit |
Supplemental Keywords:
RFA, Health, Air, Risk Assessments, particulate matter, lung disease, long term exposure, epidemiological studies, PM, toxicologyProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832414 UC Davis Center for Children's Environmental Health and Disease Prevention Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832414C001 Project 1 -- Pulmonary Metabolic Response
R832414C002 Endothelial Cell Responses to PM—In Vitro and In Vivo
R832414C003 Project 3 -- Inhalation Exposure Assessment of San Joaquin Valley Aerosol
R832414C004 Project 4 -- Transport and Fate Particles
R832414C005 Project 5 -- Architecture Development and Particle Deposition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2010 Progress Report
- 2009 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
2 journal articles for this subproject
Main Center: R832414
128 publications for this center
64 journal articles for this center
