Grantee Research Project Results
2009 Progress Report: Toxicological Evaluation of Realistic Emission Source Aerosol (TERESA): Investigation of Vehicular Emissions
EPA Grant Number: R832416C005Subproject: this is subproject number 005 , established and managed by the Center Director under grant R832416
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Health Effects Institute (2015 - 2020)
Center Director: Greenbaum, Daniel S.
Title: Toxicological Evaluation of Realistic Emission Source Aerosol (TERESA): Investigation of Vehicular Emissions
Investigators: Koutrakis, Petros , Godleski, John J.
Institution: Harvard University
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2011)
Project Period Covered by this Report: August 1, 2008 through July 31,2009
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
- Exposures to fresh and to photochemically oxidized mobile source emissions will induce cardiovascular responses in normal animals;
- Atmospheric photochemical processes enhance the toxicity of gases and particles emitted from motor vehicles, and;
- Animal models of susceptible populations (e.g., spontaneously hypertensive rats) will have greater biological responses to particles originating from motor vehicles than the corresponding normal animal model.
Progress Summary:
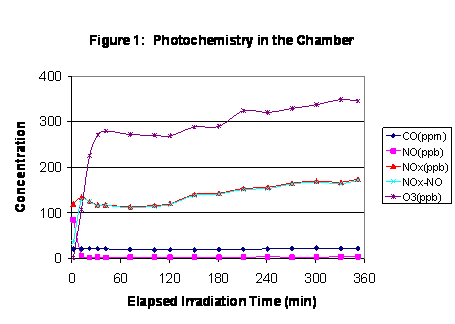
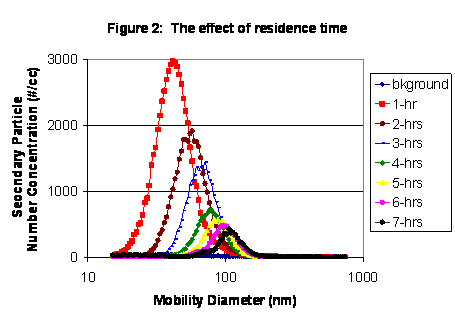
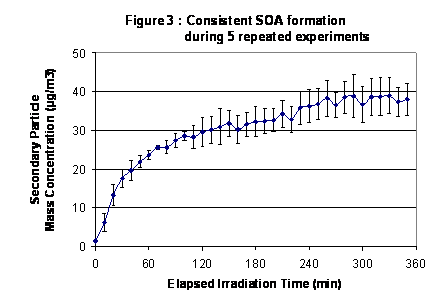
|
Baseline Seed Aerosol
Concentration (μg/m3)
|
CO Concentration
(ppm)
|
Residence Time
(min)
|
Secondary Particle Mass Generated (μg/m3)
|
|
12.5
|
5
|
50
|
54.5
|
|
26.0
|
20
|
50
|
108.8
|
|
9.5
|
5
|
100
|
51.5
|
|
12.5
|
20
|
100
|
99.1
|
|
Scenario
|
Seed PM (μg/m3)
|
PM (μg/m3)
|
EC/OC
(μg/m3)
|
BIOLOGICAL RESPONSES
|
||
|
In Vivo Chemiluminescence
|
Total BAL Cells
(x106)
|
Total BAL PMNs
(x105)
|
||||
|
Primary PM
|
298*
|
298
|
N/A
|
No heart or lung difference from control
Lung: 2.3±1.3
|
No difference from control
0.4±0.8
|
No difference from control
0.0±0.1
|
|
Secondary PM
|
142*
|
212
|
0.5/6.9
|
Significant lung ↑↑↑ from control and Primary PM
15.7±6.0**
|
Significant ↑↑ from control and Primary PM
4.9±1.1**
|
Significant ↑ from control and Primary PM
3.7±1.1**
|
|
Compound
|
D (cm2/s)*
|
Penetration (%)
|
|
Benzene
|
0.125
|
17.7
|
|
Toluene
|
0.115
|
18.9
|
|
Ethylbenzene
|
0.107
|
21.5
|
|
m/p Xylene
|
0.107
|
21.2
|
|
o Xylene
|
0.107
|
19.3
|
Future Activities:
References:
Journal Articles:
No journal articles submitted with this report: View all 9 publications for this subprojectSupplemental Keywords:
traffic related particles, acute cardiovascular effects, primary and secondary particle effects, RFA, Health, Air, Scientific Discipline, Health Risk Assessment, Risk Assessments, particulate matter, Environmental Chemistry, Toxicology, biological mechanisms, chemical characteristics, autonomic dysfunction, biological mechanism , airborne particulate matter, cardiovascular vulnerability, chemical composition, animal model, oxidative stress, ambient particle health effects, PM, atmospheric particulate matter, automobile exhaust, ambient air quality, concentrated ambient particulates (CAPs), human health effects, traffic related particulate matterRelevant Websites:
http://www.hsph.harvard.edu/epacenter/![]()
Progress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832416 Health Effects Institute (2015 - 2020) Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832416C001 Cardiovascular Responses in the Normative Aging Study: Exploring the Pathways of Particle Toxicity
R832416C002 Cardiovascular Toxicity of Concentrated Ambient Fine, Ultrafine and Coarse Particles in Controlled Human Exposures
R832416C003 Assessing Toxicity of Local and Transported Particles Using Animal Models Exposed to CAPs
R832416C004 Cardiovascular Effects of Mobile Source Exposures: Effects of Particles and Gaseous Co-pollutants
R832416C005 Toxicological Evaluation of Realistic Emission Source Aerosol (TERESA): Investigation of Vehicular Emissions
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
9 journal articles for this subproject
Main Center: R832416
206 publications for this center
199 journal articles for this center
