Grantee Research Project Results
Final Report: Assessing Toxicity of Local and Transported Particles Using Animal Models Exposed to CAPs
EPA Grant Number: R832416C003Subproject: this is subproject number 003 , established and managed by the Center Director under grant R832416
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Health Effects Institute (2015 - 2020)
Center Director: Greenbaum, Daniel S.
Title: Assessing Toxicity of Local and Transported Particles Using Animal Models Exposed to CAPs
Investigators: Godleski, John J. , Koutrakis, Petros
Institution: Harvard University
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2011)
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
Summary/Accomplishments (Outputs/Outcomes):
| Measurement | Early Morning Mean ± SE | Mid-Day Mean ± SE | p value |
| CAPs Mass | 465.9 ± 42.9 | 425.6 ± 38.2 | 0.233 |
| *Elemental Carbon | 21.7 ± 2.1 | 16.8 ± 1.9 | 0.020 |
| Organic Carbon | 82.7 ± 6.6 | 83.6 ± 6.1 | 0.910 |
| Total Carbon | 104.4 ± 8.3 | 100.3 ± 7.6 | 0.652 |
| Sodium | 8.5 ± 2.6 | 9.2 ± 1.5 | 0.348 |
| *Magnesium | 1.12 ± 0.15 | 1.34 ± 0.15 | 0.015 |
| Aluminum | 3.3 ± 0.4 | 3.7 ± 0.4 | 0.251 |
| Silicon | 8.9 ± 0.9 | 9.8 ± 0.9 | 0.329 |
| Sulfur | 38.7 ± 5.5 | 44.3 ± 6.1 | 0.243 |
| Chlorine | 6.0 ± 1.8 | 8.3 ± 2.9 | 0.136 |
| Potassium | 2.7 ± 0.2 | 3.0 ± 0.2 | 0.167 |
| Calcium | 5.8 ± 0.7 | 5.9 ± 0.4 | 0.946 |
| Titanium | 0.43 ± 0.04 | 0.44 ± 0.05 | 0.713 |
| Vanadium | 0.25 ± 0.20 | 0.04 ± 0.01 | 0.280 |
| *Iron | 11.4 ± 1.1 | 9.3 ± 0.7 | 0.039 |
| Nickel | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.078 |
| *Copper | 0.41 ± 0.07 | 0.25 ± 0.02 | 0.021 |
| Zinc | 0.91 ± 0.08 | 0.95 ± 0.10 | 0.709 |
| Lead | 0.14 ± 0.03 | 0.19 ± 0.07 | 0.587 |
| *Elemental Carbon percent of mass | 5.1 ± 0.4 | 4.0 ± 0.3 | 0.007 |
| *Organic Carbon percent of mass | 20.4 ± 1.1 | 22.4 ± 1.3 | 0.049 |
| Total Carbon percent of mass | 25.5 ± 1.4 | 26.4 ± 1.5 | 0.468 |
| Sodium percent of mass | 2.6 ± 0.5 | 2.9 ± 0.5 | 0.165 |
| *Magnesium percent of mass | 0.31 ± 0.04 | 0.40 ± 0.05 | 0.006 |
| Aluminum percent of mass | 0.91 ± 0.11 | 1.02 ± 0.11 | 0.072 |
| *Silicon percent of mass | 2.52 ± 0.27 | 2.86 ± 0.27 | 0.040 |
| *Sulfur percent of mass | 7.66 ± 0.53 | 8.97 ± 0.70 | 0.029 |
| Chlorine percent of mass | 1.65 ± 0.49 | 2.44 ± 0.70 | 0.066 |
| *Potassium percent of mass | 0.73 ± 0.06 | 0.83 ± 0.05 | 0.008 |
| Calcium percent of mass | 1.62 ± 0.19 | 1.81 ± 0.20 | 0.084 |
| Titanium percent of mass | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.519 |
| Vanadium percent of mass | 0.08 ± 0.06 | 0.01 ± 0.00 | 0.285 |
| Iron percent of mass | 3.0 ± 0.3 | 2.7 ± 0.2 | 0.081 |
| *Nickel percent of mass | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.036 |
| *Copper percent of mass | 0.10 ± 0.02 | 0.07 ± 0.01 | 0.030 |
| Zinc percent of mass | 0.24 ± 0.02 | 0.27 ± 0.03 | 0.343 |
| Lead percent of mass | 0.04 ± 0.06 | 0.06 ± 0.03 | 0.431 |
| Respiratory Parameter | Difference Mean ± SE | P value |
| Frequency | 20.7 ± 4.52 | <0.0001 |
| TV | 0.06 ± 0.20 | NS |
| Ti | -0.03 ± 0.01 | 0.0001 |
| Te | -0.02 ± 0.00 | <0.0001 |
| Penh | -0.22 ± 0.05 | <0.0001 |
| EIP | 0.26 ± 0.31 | NS |
| EEP | -16.4 ± 4.37 | 0.0002 |
| EF50 | 0.51 ± 1.27 | NS |
| PEF | 1.88 ±2.05 | NS |
| PIF | 4.23 ± 2.39 | NS |
| SITE | Effect Tested | CAPs-Sham Difference Mean ± SE | P value |
| Heart | CAPs Effect All Exposures | 5.47 ± 4.20 | 0.197 |
| CAPs Effect Different for 3 Species | -- | 0.644 | |
| CAPs Effects Different for Early Morning vs Mid-day | -- | 0.366 | |
| Lung | CAPs Effect All Exposures | 10.28 ± 3.07 | 0.001 |
| CAPs Effect Different for 3 Species | -- | 0.640 | |
| Early Morning Exposure | 12.28 ± 4.41 | 0.007 | |
| Mid-Day Exposure | 8.38 ± 4.31 | 0.055 | |
| CAPs Effects Different for Early Morning vs Mid-day | -- | 0.529 |
| Element/Component | Estimate ± SE | P value |
| CAPs Mass | 0.012±0.005 | 0.027 |
| Organic Carbon | 0.106±0.038 | 0.0064 |
| Elemental Carbon | 0.298±0.121 | 0.016 |
| Aluminum | 1.573±0.739 | 0.036 |
| Silicon | 0.577±0.267 | 0.034 |
| Sulfur | 0.132±0.061 | 0.033 |
| Iron | 0.509±0.218 | 0.022 |
| RAT STRAIN | Parameter | Time Trend Type | Difference in Time Trend between AM and PM | Difference in Time Trend among particle count concentrations | ||
| Overall | AM only | PM only | ||||
| WKY | Systolic Pressure | Linear (<0.0001)* | YES (0.0054) | YES (0.0133) | NO | Borderline (0.0509) |
| Diastolic Pressure | Linear (0.0005) | YES (0.0068) | YES (0.0004) | NO | YES (0.0008) | |
| Mean Pressure | Linear (<0.0001) | YES (0.0119) | YES (0.0011) | NO | YES (0.0032) | |
| Pulse Pressure | Linear (<0.0001) | NO | NO | NO | NO | |
| Heart Rate | Quadratic (<0.0001) | NO | Borderline (0.0511) | NO | YES (0.0035) | |
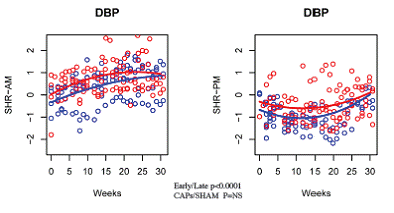
| SHR | Systolic Pressure | Quadratic (0.0004) | YES (<0.0001) | Borderline (0.0578) | NO | YES (0.0028) |
| Diastolic Pressure | Quadratic (0.0014) | YES (<0.0001) | NO | NO | YES (0.0145) | |
| Mean Pressure | Quadratic (0.0003) | YES (<0.0001) | NO | NO | YES (0.0071) | |
| Pulse Pressure | Quadratic (<0.0001) | YES (<0.0001) | NO | NO | YES (0.0257) | |
| Heart Rate | Quadratic (<0.0001) | YES (0.0001) | YES (0.0030) | YES (0.0473) | YES (0.0092) | |
References:
Journal Articles on this Report : 9 Displayed | Download in RIS Format
| Other subproject views: | All 14 publications | 12 publications in selected types | All 12 journal articles |
|---|---|---|---|
| Other center views: | All 206 publications | 199 publications in selected types | All 199 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Bartoli CR, Wellenius GA, Coull BA, Akiyama I, Diaz EA, Lawrence J, Okabe K, Verrier RL, Godleski JJ. Concentrated ambient particles alter myocardial blood flow during acute ischemia in conscious canines. Environmental Health Perspectives 2009;117(3):333-337. |
R832416 (2009) R832416C003 (2009) R832416C003 (Final) |
|
|
|
Bartoli CR, Wellenius GA, Diaz EA, Lawrence J, Coull BA, Akiyama I, Lee LM, Okabe K, Verrier RL, Godleski JJ. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environmental Health Perspectives 2009;117(3):361-366. |
R832416 (2008) R832416 (2009) R832416C003 (2009) R832416C003 (Final) R827353 (Final) |
|
|
|
Bartoli CR, Nadar MM, Godleski JJ. Capsule thickness correlates with vascular density and blood flow within foreign-body capsules surrounding surgically implanted subcutaneous devices. Artificial Organs 2010;34(10):857-861. |
R832416 (Final) R832416C003 (Final) R827353 (Final) R831917 (Final) |
Exit |
|
|
Bartoli CR, Godleski JJ, Verrier RL. Mechanisms mediating adverse effects of air pollution on cardiovascular hemodynamic function and vulnerability to cardiac arrhythmias. Air Quality, Atmosphere & Health 2011;4(1):53-63. |
R832416 (Final) R832416C003 (Final) R827353 (Final) R831917 (Final) |
Exit Exit |
|
|
Clougherty JE, Rossi CA, Lawrence J, Long MS, Diaz EA, Lim RH, McEwen B, Koutrakis P, Godleski JJ. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environmental Health Perspectives 2010;118(6):769-775. |
R832416 (Final) R832416C003 (Final) |
|
|
|
Ghelfi E, Wellenius GA, Lawrence J, Millet E, Gonzalez-Flecha B. Cardiac oxidative stress and dysfunction by fine concentrated ambient particles (CAPs) are mediated by angiotensin-II. Inhalation Toxicology 2010;22(11):963-972. |
R832416 (Final) R832416C003 (Final) |
Exit |
|
|
Imrich A, Ning YY, Lawrence J, Coull B, Gitin E, Knutson M, Kobzik L. Alveolar macrophage cytokine response to air pollution particles:oxidant mechanisms. Toxicology and Applied Pharmacology 2007;218(3):256-264. |
R832416 (2008) R832416 (Final) R832416C003 (Final) |
Exit Exit Exit |
|
|
Nikolov MC, Coull BA, Catalano PJ, Diaz E, Godleski JJ. Statistical methods to evaluate health effects associated with major sources of air pollution: a case-study of breathing patterns during exposure to concentrated Boston air particles. Journal of the Royal Statistical Society Series C-Applied Statistics 2008;57(3):357-378. |
R832416 (Final) R832416C003 (Final) |
Exit |
|
|
Nikolov MC, Coull BA, Catalano PJ, Godleski JJ. Multiplicative factor analysis with a latent mixed model structure for air pollution exposure assessment. Environmetrics 2011;22(2):165-178. |
R832416 (2009) R832416 (Final) R832416C003 (2009) R832416C003 (Final) |
Exit |
Supplemental Keywords:
RFA, Health, Air, Scientific Discipline, Health Risk Assessment, Risk Assessments, particulate matter, Environmental Chemistry, autonomic dysfunction, biological mechanism , biological mechanisms, chemical characteristics, airborne particulate matter, human exposure, chemical composition, mobile sources, cardiovascular vulnerability, oxidative stress, ambient particle health effects, automobile exhaust, atmospheric particulate matter, human health effects, human health riskProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832416 Health Effects Institute (2015 - 2020) Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832416C001 Cardiovascular Responses in the Normative Aging Study: Exploring the Pathways of Particle Toxicity
R832416C002 Cardiovascular Toxicity of Concentrated Ambient Fine, Ultrafine and Coarse Particles in Controlled Human Exposures
R832416C003 Assessing Toxicity of Local and Transported Particles Using Animal Models Exposed to CAPs
R832416C004 Cardiovascular Effects of Mobile Source Exposures: Effects of Particles and Gaseous Co-pollutants
R832416C005 Toxicological Evaluation of Realistic Emission Source Aerosol (TERESA): Investigation of Vehicular Emissions
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2010
- 2009 Progress Report
- 2008 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
12 journal articles for this subproject
Main Center: R832416
206 publications for this center
199 journal articles for this center
