Grantee Research Project Results
2008 Progress Report: Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
EPA Grant Number: R832415C002Subproject: this is subproject number 002 , established and managed by the Center Director under grant R832415
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Rochester PM Center
Center Director: Oberdörster, Günter
Title: Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
Investigators: Peters, Annette , Zareba, Wojciech , Utell, Mark J. , Henneberger, Alexandra , Wichmann, Heinz-Erich , Stoelzel, M , Rückerl, Regina , Phipps, Richard , Breitner, Susanne
Current Investigators: Peters, Annette , Utell, Mark J. , Zareba, Wojciech , Phipps, Richard , Wichmann, Heinz-Erich , Henneberger, Alexandra , Breitner, Susanne , Stoelzel, M , Rückerl, Regina
Institution: GSF-National Research Center for Environment and Health , University of Rochester
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
Project Period Covered by this Report: July 1, 2007 through June 30,2008
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
Our objective is to evaluate the role of key components of urban aerosol on acute phase reaction and prothrombotic states in the blood, endothelial dysfunction, cardiac function and classical cardiovascular risk factors in susceptible subgroups. We will focus on determining the effects of fresh ultrafine particles from specific sources such as traffic as well as aged aerosols in the accumulation mode which are mixtures of particles from different sources and secondary aerosols. We hypothesize that the following subgroups are susceptible to effects of ambient fine and ultrafine particles compared to control subjects: (a) Patients with coronary artery disease during rehabilitation, (b) myocardial infarction (MI) survivors, (c) diabetics, (d) individuals with enhanced inflammatory responses due to genetic polymorphisms in inflammation regulating pathways and/or in detoxifying pathways.
Approach:
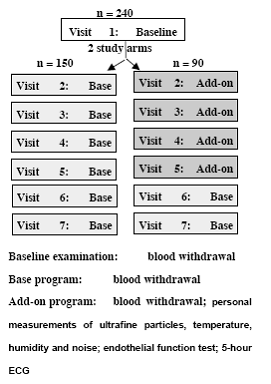
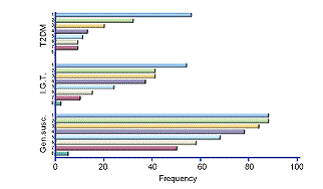
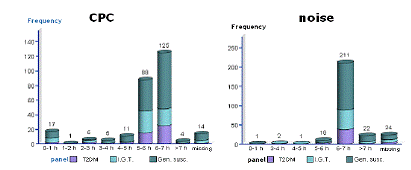
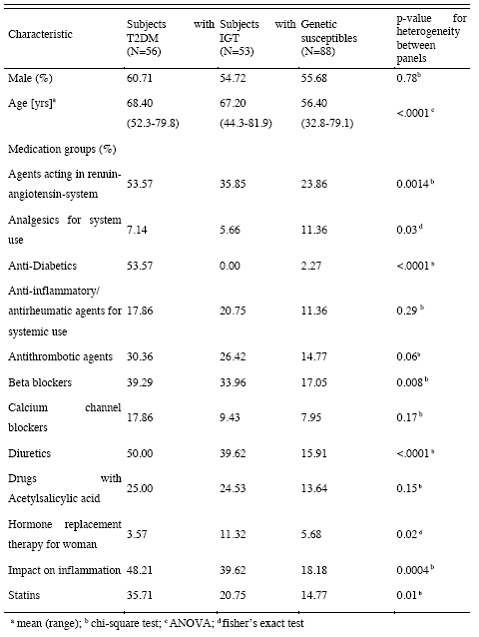
In Rochester 80 patients taking part in a cardiac rehabilitation program will be studied. In Augsburg, panel studies enrolling a total of 400 subjects will be conducted including 100 subjects per group b-d. A random sample of the population of Augsburg will be screened for genetic predispositions for enhanced inflammatory responses and slower detoxification pathways to select participants with genetic polymorphisms as well as healthy controls. In a sub-study, data on 24 hour ECG Holter monitoring, a time-activity diary including traffic exposures and stress level, and continuous personal exposure to the total particle number concentrations will be collected in 30 participants of each group. Outcome measures will be determined by the Vascular & Inflammation Core and ECG parameters will be provided by the Cardiac Core. The number concentration of ultra fine particles, particle size distributions and continuous particle mass measurements are conducted in Rochester, NY. Ambient air pollution in Augsburg will include continuous particle mass measurements, particle size distributions separated into volatile and non-volatile fractions, particle surface, elemental and organic carbon, sulphates and nitrates. PM associated oxidative stress will be measured in Rochester and Augsburg by the Aerosol Generation and Analysis Core. In collaboration with Core 1 source apportionment analyses will be conducted.
Progress Summary:
Expected Results:
The studies will give evidence on health effects of key characteristics of the urban aerosol such as oxidative stress and on the role of sources in urban areas. In addition, they will assess the potential role of ambient air pollution concentrations on smaller time-scales than 24 hours.
Future Activities:
Journal Articles on this Report : 2 Displayed | Download in RIS Format
| Other subproject views: | All 19 publications | 18 publications in selected types | All 18 journal articles |
|---|---|---|---|
| Other center views: | All 191 publications | 157 publications in selected types | All 144 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Pitz M, Birmili W, Schmid O, Peters A, Wichmann HE, Cyrys J. Quality control and quality assurance for particle size distribution measurements at an urban monitoring station in Augsburg, Germany. Journal of Environmental Monitoring 2008;10(9):1017-1024. |
R832415 (2007) R832415 (2008) R832415 (2010) R832415 (Final) R832415C002 (2006) R832415C002 (2008) R832415C002 (2010) R832415C002 (2011) |
Exit |
|
|
Yue W, Stolzel M, Cyrys J, Pitz M, Heinrich J, Kreyling WG, Wichmann H-E, Peters A, Wang S, Hopke PK. Source apportionment of ambient fine particle size distribution using positive matrix factorization in Erfurt, Germany. Science of the Total Environment 2008;398(1-3):133-144. |
R832415 (2007) R832415 (2008) R832415 (2010) R832415 (2011) R832415 (Final) R832415C001 (2008) R832415C001 (2010) R832415C001 (2011) R832415C002 (2006) R832415C002 (2008) R832415C002 (2010) R832415C002 (2011) R827354 (Final) R834797 (2016) |
Exit Exit Exit |
Supplemental Keywords:
Mechanisms, biomarker, gene-environment interaction, personal monitoring,, Health, RFA, Scientific Discipline, Air, PHYSICAL ASPECTS, Health Risk Assessment, Physical Processes, Risk Assessments, particulate matter, Epidemiology, human exposure, long term exposure, aersol particles, atmospheric particles, exposure, PM, ambient particle health effects, atmospheric aerosol particles, atmospheric particulate matter, cardiovascular disease, human health risk, acute cardiovascular effectsProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832415 Rochester PM Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832415C001 Characterization and Source Apportionment
R832415C002 Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
R832415C003 Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
R832415C004 Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
R832415C005 Ultrafine Particle Cell Interactions In Vitro: Molecular Mechanisms Leading To Altered Gene Expression in Relation to Particle Composition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2011 Progress Report
- 2010 Progress Report
- 2009 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
18 journal articles for this subproject
Main Center: R832415
191 publications for this center
144 journal articles for this center
