Grantee Research Project Results
2016 Progress Report: Vascular MAPs: Vascular and Neurovascular Tissue Models
EPA Grant Number: R835737C004Subproject: this is subproject number 004 , established and managed by the Center Director under grant R835737
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Human Models for Analysis of Pathways (H MAPs) Center
Center Director: Murphy, William L
Title: Vascular MAPs: Vascular and Neurovascular Tissue Models
Investigators: Sheibani, Nader
Institution: University of Wisconsin - Madison
EPA Project Officer: Aja, Hayley
Project Period: December 1, 2014 through November 30, 2018 (Extended to November 30, 2019)
Project Period Covered by this Report: December 1, 2015 through November 30,2016
RFA: Organotypic Culture Models for Predictive Toxicology Center (2013) RFA Text | Recipients Lists
Research Category: Chemical Safety for Sustainability
Objective:
The objectives of this project are to:
1) Develop human models of vascular and neurovascular organotypic cultures.
2) Identify adverse outcome pathways associated with environmental toxins.
3) Define the critical elements for an organotypic response, critical cell types, and tissue architecture.
4) Leverage synthetic matrices and microscale systems to achieve robust tissue assembly and enable high throughput analysis.
5) Determine broad applicability of the model for translational research in predictive toxicology.
Progress Summary:
strong>1) Develop human models of vascular and neurovascular organotypic cultures.
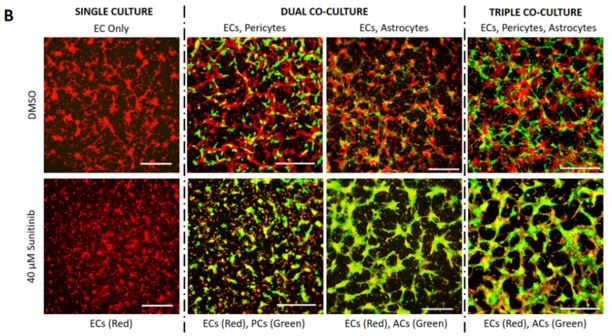
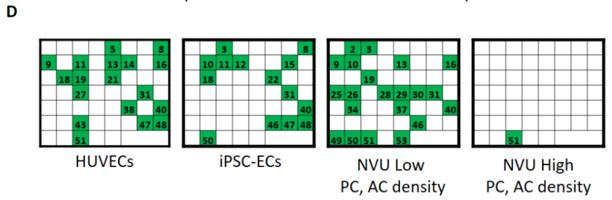
We have co-cultured human iPSC-derived endothelial cells (iPSC-EC) with human brain-derived pericytes (PC) and human iPSC-derived astrocytes (iPSC-AC) to create an in-vitro neurovascular unit model (Figure 1). Vascular morphogenesis occurs differentially depending on whether iPSC-EC are cultured alone on defined synthetic extracellular matrices, or whether pericytes and astrocytes are included in culture. We now are capable of modifying the ratio of endothelial cells and support cells in culture, as well as the composition of the defined synthetic matrix. These tools lend themselves to potentially modelling vasculature in different regions of the central nervous system, and modelling neurovasculature during varying stages of development. We have applied a screen of 38 blinded compounds to the neurovascular unit model as a testbed demonstration of a chemical toxicity screen.
2) Identify adverse outcome pathways associated with environmental toxins.
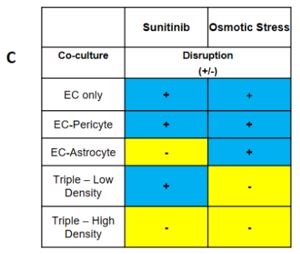
Our proposed workflow in utilizing the neurovascular unit model is capable of identifying differential effects of varying toxic exposures on the neurovascular unit. As proofs of concept, we exposed iPSC-EC, alone or in combination with PC and iPSC-AC, with high glucose representing diabetic hyperglycemia and associated osmotic stress, as well as VEGF inhibitor Sunitinib Malate. We observed differential responses to both inhibitors depending on cells present. We can apply panels of chemicals to the neurovascular co-cultures and expect to use techniques such as PCR and RNA-seq to identify active pathways in the aggregate neurovascular cell population that are specific for inhibitors selected from the panel
3) Define the critical elements for an organotypic response, critical cell types, and tissue architecture.
Our proposed workflow in utilizing the neurovascular unit model is capable of identifying individual cell types in the model neurovasculature that are affected by toxic insults. This was demonstrated by differential responses by EC-Pericyte co-cultures, EC-Astrocyte co-cultures, and co-cultures of all three cell types to high glucose and Sunitinib Malate. Our results suggest that in the neurovascular unit model, iPSC-EC, PC, and iPSC-AC are adversely affected by high D-glucose and metabolically inactive L-glucose, and that astrocytes are predominantly responsible for protecting the integrity of the neurovascular unit from Sunitinib (Figure 1). We will apply this workflow in future studies, whereby blinded chemical compound panels are applied to neurovascular co-cultures, compounds of interest are identified, and selected compounds are applied to individual cell types and co-cultures of two cell types to identify cell types that are active in protecting the neurovascular unit, or are adversely affected by applied chemicals.
4) Leverage synthetic matrices and microscale systems to achieve robust tissue assembly and enable high throughput analysis.
We are working to adapt the neurovascular unit cells and three-dimensional (3D) encapsulation techniques to generate neurovascular models in 3D. We recently have begun utilizing microfluidic devices created by the Cancer MAPS center to generate cell networks that are immediately amenable to 3D perfusion tests, as well as tests to quantify features of network morphology and physical interactions between differential cell types in a 3D space. We are beginning to apply vascular inhibitors such as Sunitinib to the cells in order to gauge those effects on vascular morphology, and aim to expand tests to other known and unknown compounds to screen larger chemical libraries.
5) Determine broad applicability of the model for translational research in predictive toxicology.
Our lab has developed various preclinical models of ocular angiogenesis and these have been used to study adverse mechanisms that lead to pathological neovascularization including ischemia, diabetes, and inflammation. Identification of various classes of chemicals with potential antiangiogenic activity using our screening platform may have clinical translational values for many diseases with a neurovascular component, and can be evaluated in these models. Additionally, we expect to develop the capability to utilize cells from multiple sources, including patient-specific cell sources. We also are developing materials for utilization on in-vivo and organ-level vascularization experiments, including mouse implantation models and vascular explant sprouting models.
Significance. The results of our studies demonstrate our ability to synthesize well-defined synthetic cell culture substrates that efficiently support capillary morphogenesis by iPSC-EC. These substrates are adaptable for use in high-throughput screening systems and are amenable to various modifications that impact angiogenesis. Exploration of co-culture protocols and adaptation to microfluidic systems will accelerate the elucidation of regulatory mechanisms that maintain vascular integrity and function and how these are disrupted by various toxic chemicals with potential adverse effects on the vasculature and organ dysfunction. Discoveries here may lead to preventative measures and therapeutic pathways that lead to reduced impact of neurological diseases such as Alzheimer’s disease, Parkinson’s disease, ALS, and Multiple Sclerosis.
Future Activities:
Our future plans will focus on further expanding the capabilities of the 2D vascular screen to model the effects of chemical toxins on different regions of the central nervous system and different stages of neurovascular development. We also will apply gene expression analysis techniques in collaboration with the bioinformatics arm of the hMAPS center to identify active pathways using the neurovascular model, and determine the efficacy of applying the techniques on different co-culture ratios of neurovascular cells. In addition, we will continue optimizing 3D encapsulation techniques to use fluid perfusion and co-culture assembly in 3D as readouts of neurovascular integrity and function.
Journal Articles on this Report : 11 Displayed | Download in RIS Format
| Other subproject views: | All 21 publications | 17 publications in selected types | All 17 journal articles |
|---|---|---|---|
| Other center views: | All 215 publications | 82 publications in selected types | All 81 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Belair DG, Miller MJ, Wang S, Darjatmoko SR, Binder BYK, Sheibani N, Murphy WL. Differential regulation of angiogenesis using degradable VEGF-binding microspheres. Biomaterials 2016;93:27-37. |
R835737 (2015) R835737 (2016) R835737 (2017) R835737C004 (2015) R835737C004 (2016) |
Exit |
|
|
Dinu D, Chu C, Veith A, Lingappan K, Couroucli X, Jefcoate CR, Sheibani N, Moorthy B. Mechanistic role of cytochrome P450 (CYP)1B1 in oxygen-mediated toxicity in pulmonary cells:a novel target for prevention of hyperoxic lung injury. Biochemical and Biophysical Research Communications 2016;476(4):346-351. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit |
|
|
Farnoodian M, Halbach C, Slinger C, Pattnaik BR, Sorenson CM, Sheibani N. High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression. American Journal of Physiology-Cell Physiology 2016;311(3):C418-C436. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit |
|
|
Ghanian Z, Staniszewski K, Jamali N, Sepehr R, Wang S, Sorenson CM, Sheibani N, Ranji M. Quantitative assessment of retinopathy using multi-parameter image analysis. Journal of Medical Signals and Sensors 2016;6(2):71-80. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit |
|
|
Ren J, Liu Z, Wang Q, Giles J, Greenberg J, Sheibani N, Kent KC, Liu B. Andrographolide ameliorates abdominal aortic aneurysm progression by inhibiting inflammatory cell infiltration through downregulation of cytokine and integrin expression. Journal of Pharmacology and Experimental Therapeutics 2016;356(1):137-147. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit Exit |
|
|
Saghiri MA, Orangi J, Asatourian A, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis part III: (Ti, Li, Ce, As, Hg, Va, Nb and Pb). Critical Reviews in Oncology/Hematology 2016;98:290-301. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit Exit Exit |
|
|
Saghiri MA, Asatourian A, Garcia-Godoy F, Sheibani N. The role of angiogenesis in implant dentistry part I: review of titanium alloys, surface characteristics and treatments. Medicina Oral, Patología Oral y Cirugía Bucal 2016;21(4):e514-e525. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit Exit Exit |
|
|
Saghiri MA, Asatourian A, Garcia-Godoy F, Sheibani N. The role of angiogenesis in implant dentistry part II: the effect of bone-grafting and barrier membrane materials on angiogenesis. Medicina Oral, Patología Oral y Cirugía Bucal 2016;21(4):e526-e537. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit Exit |
|
|
Saghiri MA, Asatourian A, Garcia-Godoy F, Sheibani N. Effect of biomaterials on angiogenesis during vital pulp therapy. Dental Materials Journal 2016;35(5):701-709. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit Exit Exit |
|
|
Saghiri MA, Orangi J, Asatourian A, Gutmann JL, Garcia-Godoy F, Lotfi M, Sheibani N. Calcium silicate-based cements and functional impacts of various constituents. Dental Materials Journal 2017;36(1):8-18. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit |
|
|
Ye G-J, Budzynski E, Sonnentag P, Nork TM, Sheibani N, Gurel Z, Boye SL, Peterson JJ, Boye SE, Hauswirth WW, Chulay JD. Cone-specific promoters for gene therapy of achromatopsia and other retinal diseases. Human Gene Therapy 2016;27(1):72-82. |
R835737 (2016) R835737 (2017) R835737C004 (2016) |
Exit Exit |
Progress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R835737 Human Models for Analysis of Pathways (H MAPs) Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R835737C001 Liver MAPs
R835737C002 Brain MAPs
R835737C003 Cancer MAPs: A 3D Organotypic Microfluidic Culture System to
Identify Chemicals that Impact Progression and Development of Breast Cancer
R835737C004 Vascular MAPs: Vascular and Neurovascular Tissue Models
R835737C005 Pathway Analysis Core
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
17 journal articles for this subproject
Main Center: R835737
215 publications for this center
81 journal articles for this center