Grantee Research Project Results
Final Report: Gastrointestinal TSLP in the Pathogenesis of Food Allergy
EPA Grant Number: R834064Title: Gastrointestinal TSLP in the Pathogenesis of Food Allergy
Investigators: Berin, M. Cecilia
Institution: Mount Sinai School of Medicine
EPA Project Officer: Aja, Hayley
Project Period: December 1, 2008 through November 30, 2010 (Extended to November 30, 2011)
Project Amount: $466,125
RFA: Exploratory Investigations in Food Allergy (2007) RFA Text | Recipients Lists
Research Category:
Objective:
Summary/Accomplishments (Outputs/Outcomes):
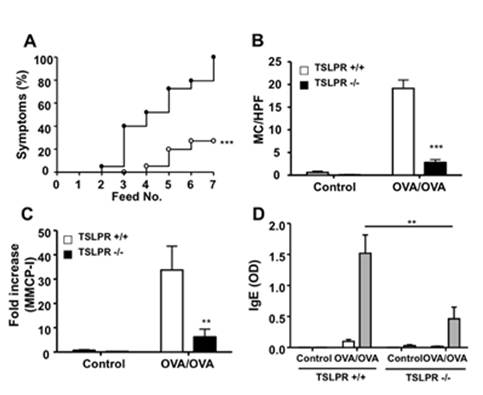
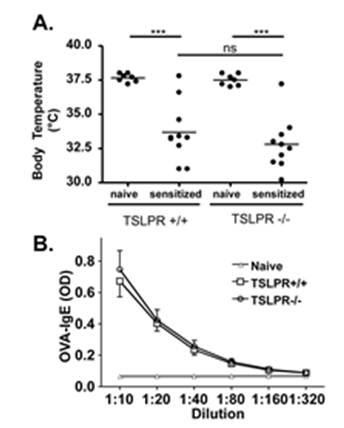
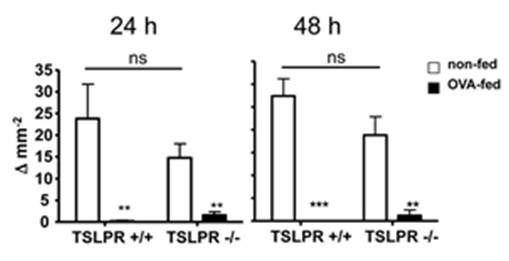
Quality Assurance:
All experiments performed using pooled tissue specimens were repeated a minimum of 3 times so that statistical analysis could be performed. Studies using in vivo responses of individual mice utilized a minimum of 5 mice per group and experiments were repeated to verify results. Raw data was examined by the principal investigator as well as the postdoctoral fellow (Ana Belen Blazquez) or technician (Wei Wang) performing the experiments.
Cytokine measurements were performed using commercially available assay kits. A range of standard curves was compared to the manufacturer’s representative results. Antigen-specific immunoglobulin levels were measured as dilution curves, with all comparisons being performed within the same plate. Positive and negative controls were included on each plate.
Crude allergen extracts (peanut, soy, rice) were prepared at the Mount Sinai School of Medicine, and verified by SDS page. Purified allergen extracts were purchased from commercial sources. Mice were purchased from National Cancer Institute (Frederick, MD) or bred at the Mount Sinai School of Medicine. All experiments were approved by the Institutional Animal Care and Use Committee.
Conclusions:
References:
Journal Articles on this Report : 10 Displayed | Download in RIS Format
| Other project views: | All 11 publications | 10 publications in selected types | All 10 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Berin MC, Mayer L. Immunophysiology of experimental food allergy. Mucosal Immunology 2009;2(1):24-32. |
R834064 (Final) |
Exit Exit |
|
|
Berin MC, Sicherer S. Food allergy: mechanisms and therapeutics. Current Opinion in Immunology 2011;23(6):794-800. |
R834064 (Final) |
Exit |
|
|
Berin MC. Mechanisms of allergic sensitization to foods: bypassing immune tolerance pathways. Immunology and Allergy Clinics of North America 2012;32(1):1-10. |
R834064 (Final) |
Exit Exit |
|
|
Berin MC, Wang W. Reduced severity of peanut-induced anaphylaxis in TLR9-deficient mice is associated with selective defects in humoral immunity. Mucosal Immunol 2013;6(1):114-121. |
R834064 (Final) |
Exit Exit |
|
|
Blazquez AB, Knight AK, Getachew H, Bromberg JS, Lira SA, Mayer L, Berin MC. A functional role for CCR6 on proallergic T cells in the gastrointestinal tract. Gastroenterology 2010;138(1):275-284. |
R834064 (2009) R834064 (Final) |
Exit Exit Exit |
|
|
Blazquez AB, Mayer L, Berin MC. Thymic stromal lymphopoietin is required for gastrointestinal allergy but not oral tolerance. Gastroenterology 2010;139(4):1301-1309. |
R834064 (2009) R834064 (Final) |
Exit Exit |
|
|
Dunkin D, Berin MC, Mayer L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. Journal of Allergy and Clinical Immunology 2011;128(6):1251-1258. |
R834064 (Final) |
Exit Exit Exit |
|
|
Lopez-Exposito I, Chicon R, Belloque J, Lopez-Fandino R, Berin MC. In vivo methods for testing allergenicity show that high hydrostatic pressure hydrolysates of β-lactoglobulin are immunologically inert. Journal of Dairy Science 2012;95(2):541-548. |
R834064 (Final) |
Exit Exit |
|
|
Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Wegrzyn A. Mechanisms underlying differential food allergy response to heated egg. Journal of Allergy and Clinical Immunology 2011;127(4):990-997. |
R834064 (Final) |
Exit Exit |
|
|
Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, Maleki SJ, Sampson HA, Berin MC. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. The Journal of Clinical Investigation 2014;124(11):4965-4975. |
R834064 (Final) |
Exit Exit |
Supplemental Keywords:
allergens, diet, health effects, susceptibility, exposureProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
