Grantee Research Project Results
Final Report: Rapid Concentration, Detection, and Quantification of Pathogens in Drinking Water
EPA Grant Number: R833840Title: Rapid Concentration, Detection, and Quantification of Pathogens in Drinking Water
Investigators: Hu, Zhiqiang , Riley, Lela K. , Lin, Mengshi
Institution: University of Missouri - Columbia
EPA Project Officer: Aja, Hayley
Project Period: May 1, 2008 through April 30, 2011 (Extended to April 30, 2013)
Project Amount: $600,000
RFA: Development and Evaluation of Innovative Approaches for the Quantitative Assessment of Pathogens and Cyanobacteria and Their Toxins in Drinking Water (2007) RFA Text | Recipients Lists
Research Category: Drinking Water , Water
Objective:
Pathogens in drinking water and their associated waterborne disease are of great concern to the public health. Numerous molecular technologies and methods have been developed and are being applied to pathogen determination in drinking water. Most of these methods, however, require sample pretreatment to concentrate the bacteria or viruses in water before analysis. A common method to accomplish this requirement is filtration using a large volume of test water. The filtration process can be time-consuming because the accumulation of organic matter in water forms a layer of cake on the filter membrane and reduces the filtration rate. This project aims to develop a lanthanum-based chemical concentration method, which can be combined with spectroscopic-based detection techniques and standard molecular methods for rapidly determining pathogens in drinking water. The objectives of this research are to: (1) evaluate a lanthanum-based colloidal destabilization method to rapidly concentrate pathogens in water; (2) determine the efficiency of fluorescence-based oxygen microrespirometry in differentiating live/dead pathogens; (3) develop and validate a new SERS-based method for pathogen detection and quantification; and (4) improve pathogen detection using lanthanum-based concentration and molecular detection methods and compare the detection efficiencies to the Environmental Protection Agency’s (EPA) existing methods.
Summary/Accomplishments (Outputs/Outcomes):
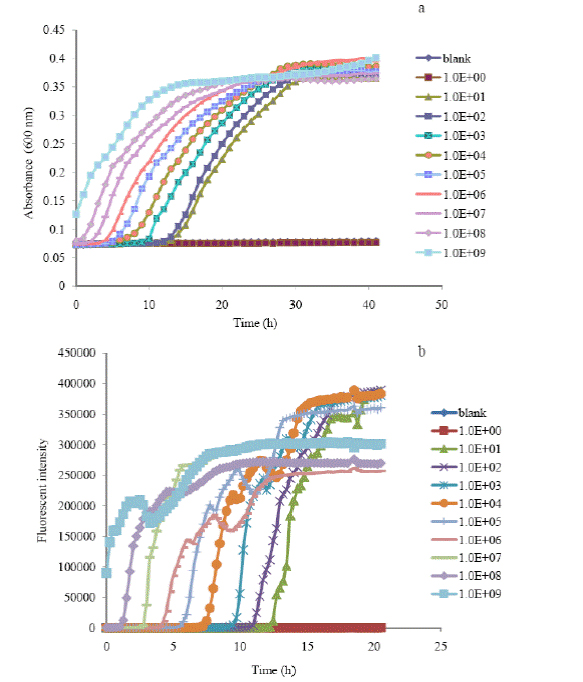
Figure 1. Time-growth profiles of absorbance (a) and oxygen flourenscence intensity (b) of E.
coli at different initial cell concentrations based on the turbidimetrec assay (a) and
microrespirometic (b) assay.
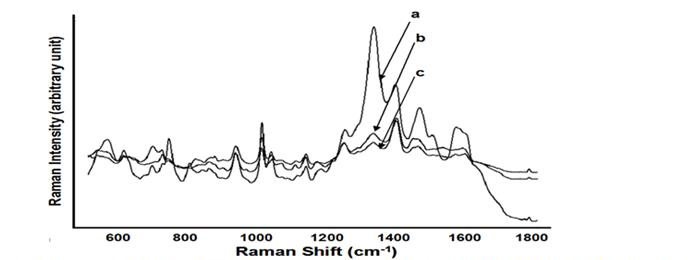
Figure 2. Average SERS spectra (n=7) acquired from 3 bacterial species with internal coating treatment
in a concentration of 109. s. epidermidis (a), E. coli K-12 (b), E. coli 0157:H7 (c).
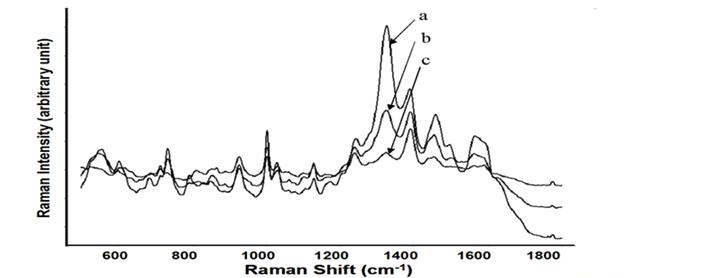
Figure 3. Average SERS spectra (n=7) acquired from S. epedermis, E. coli 0157:H7 and the 1:1 ratio
mixture with a concentration of 109: S. epdermidis (a), and the 1:1 ration mixture of S. epidermidis and
E. coli 0157:H7 (b), E. coli 0157:H7 (c).
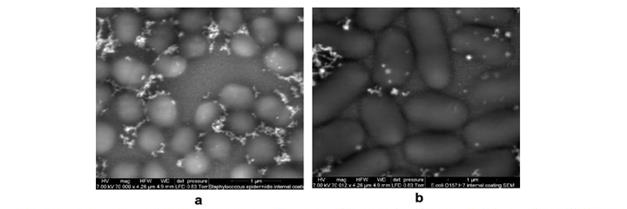
Figure 4. Scanning electron microscopy images of bacterial cells internally coated with silver
nanostructures: S. epidermidis (a), E. coli 0157:H7 (b)
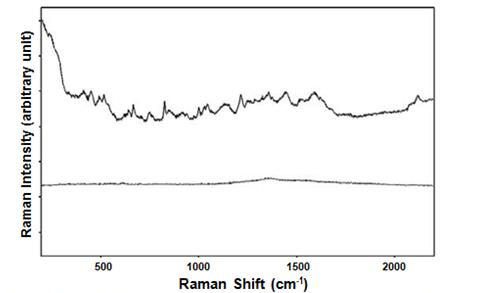
Figure 5. SERS spectrum and normal Raman spectrum of virus.
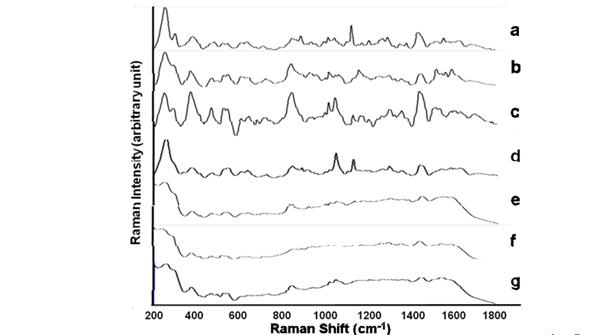
Figure 6. Average SERS spectru (n=5) acquired from 7 viruses with a titer ranging from 106 -107
in deionized water: Norovirus-MNV4 (a), Adenovirus-MAD-1 (mouse) (b), Parvovirus-MVM (mouse)
(c), Rotavirus-SA-11 (monkey)(d), Coronavirus-MVH (mouse) (e), Paramyxovirus-Sendai (mouse)
(f), Herpesvirus-MCMV (mouse) (g).
| Bacteria | Target gene | Amplicon size (bp) | Primer and Probe Sequence (5'-3') |
|---|---|---|---|
| E. coli | lacZ | 180 | Forward primer (lacZf) |
| CTTAATCGCCTTGCAGCACA | |||
| Reverse Primer (lacZr) | |||
| CAGTATCGGCCTACAGGAAGA | |||
| Probe (lac-ZTM) | |||
| ATTCGCCATTACAGGCTGCGCAA | |||
| h. pylori | ureA | 135 | Forward primer (HpyF1) |
| GGGTATTGAAGCGATGTTTCCT | |||
| Reverse primer (HpyR1) | |||
| GCTTTTTTGCCTTCGTTGATAGT | |||
| Probe (HpyP1 | |||
| AAACTCGTAACCGTGCATACCCCTATTGAG |
| Volume (mL) | Viral particles recovered (± SD) | Mean recovery (± SD) % | |
|---|---|---|---|
| Lanthanum flocs | 1000 | 5369 (± 0.62) x 104 | 70.7 (± 7.7) |
| Filtrate | 1000 | ND | ND |
| Eluate | 15 | 8.35 (± 1.38) x 104 | 103.9 (± 17.2) |
| Centrifugation | 2.35 | 5.51 (± 3.630) x 104 | 68.5 (± 44.8) |
The total number of adenovirus spiked inot the 40-L decholrinated tap water (n=3) was 8.01 (± 11.26) x 104 VP
adn the initial virus concentrataion was 2 adenovirus particles/mL. ND = Not Detected.
Conclusions:
To summarize, a new method using lanthanum-based chemical flocculation was successfully developed for PCR detection of pathogens in water samples. EDTA completely dissolved the flocs from the lanthanum-based flocculation process, with additional bacterial concentration options through two-step flocculation, filtration and centrifugation. The lanthanum-based concentration method consistently showed higher virus detection efficiency than the currently EPA-approved methods in this study. No PCR inhibition was found throughout each concentration step. Furthermore, it took less than 4 hours with the lanthanum-based concentration/membrane filtration method compared to at least 6 hours required to complete all steps in the direct filtration method using Nanoceram or 1MDS. This project presents a proof of concept implementation of the pathogen concentration procedures, which could lead to new efforts to concentrate and detect low concentrations of pathogens in water with larger volumes and mixed microbial species.
Journal Articles on this Report : 5 Displayed | Download in RIS Format
| Other project views: | All 6 publications | 5 publications in selected types | All 5 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Fan C, Hu Z, Riley LK, Purdy GA, Mustapha A, Lin M. Detecting food-and waterborne viruses by surface-enhanced Raman spectroscopy. Journal of Food Science 2010;75(5):M302-M307. |
R833840 (2009) R833840 (Final) |
Exit |
|
|
Fan C, Hu Z, Mustapha A, Lin M. Rapid detection of food-and waterborne bacteria using surface-enhanced Raman spectroscopy coupled with silver nanosubstrates. Applied Microbiology and Biotechnology 2011;92(5):1053-1061. |
R833840 (2010) R833840 (2011) R833840 (Final) |
Exit |
|
|
Zhang Y, Riley LK, Lin M, Hu Z. Lanthanum-based concentration and microrespirometric detection of microbes in water. Water Research 2010;44(11):3385-3392. |
R833840 (2009) R833840 (Final) |
Exit |
|
|
Zhang Y, Riley LK, Lin M, Hu Z. Determination of low-density Escherichia coli and Helicobacter pylori suspensions in water. Water Research 2012;46(7):2140-2148. |
R833840 (2010) R833840 (2011) R833840 (Final) |
Exit Exit |
|
|
Zhang Y, Riley LK, Lin M, Purdy GA, Hu Z. Development of a virus concentration method using lanthanum-based chemical flocculation coupled with modified membrane filtration procedures. Journal of Virological Methods 2013;190(1-2):41-48. |
R833840 (Final) |
Exit |
Supplemental Keywords:
Drinking water, exposure, waterborne pathogens, microbiology, monitoring, measurement methods, physical processes, health effects, field-based detection, lanthanum, respirometry, Surface Enhanced Raman Spectroscopy, nanotechnology, biotechnology, RFA, Health, Ecosystem Protection/Environmental Exposure & Risk, Scientific Discipline, PHYSICAL ASPECTS, Water, Health Risk Assessment, Physical Processes, Risk Assessments, Environmental Chemistry, Monitoring/Modeling, Drinking Water, Environmental Monitoring, microbial contamination, E. Coli, virulence factor activity relationships, human exposure, measurement, microbiological organisms, microorganism, measurement , microbial risk assessment, microbial risk management, monitoring, bacteria monitoring, drinking water contaminants, exposure, assessment technology, other - risk assessment, virulence factor biochip, human health risk, detection, exposure and effectsProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
