Grantee Research Project Results
2009 Progress Report: Improved Prediction of In-Cloud Biogenic SOA: Experiments and CMAQ Model Refinements
EPA Grant Number: R833751Title: Improved Prediction of In-Cloud Biogenic SOA: Experiments and CMAQ Model Refinements
Investigators: Turpin, Barbara , Seitzinger, Sybil
Institution: Rutgers
EPA Project Officer: Chung, Serena
Project Period: November 1, 2007 through August 31, 2010 (Extended to October 31, 2011)
Project Period Covered by this Report: September 1, 2008 through August 31,2009
Project Amount: $598,544
RFA: Sources and Atmospheric Formation of Organic Particulate Matter (2007) RFA Text | Recipients Lists
Research Category: Particulate Matter , Air Quality and Air Toxics , Air
Objective:
1) Develop mechanistic/ kinetic data needed to simulate in-cloud formation of secondary organic aerosol (SOA) in the presence of HNO3, 2) Identify conditions for which predicted in-cloud SOA formed from isoprene decreases with reductions in interstitial concentrations of ·OH and HNO3 (atmospheric oxidants with anthropogenic precursors), and 3) Incorporate an in-cloud SOA formation pathway into the Community Multiscale Air Quality (CMAQ) model and explore the magnitude of in-cloud SOA formation through a limited set of model simulations.
Progress Summary:
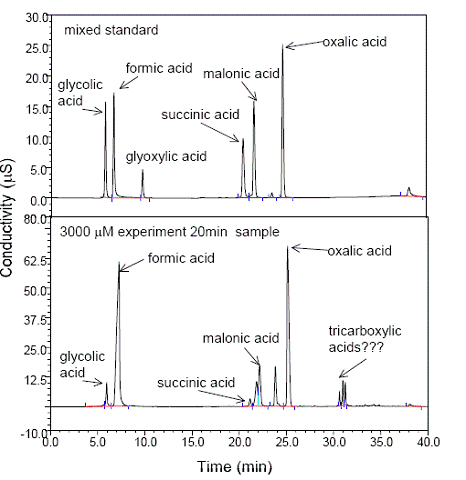
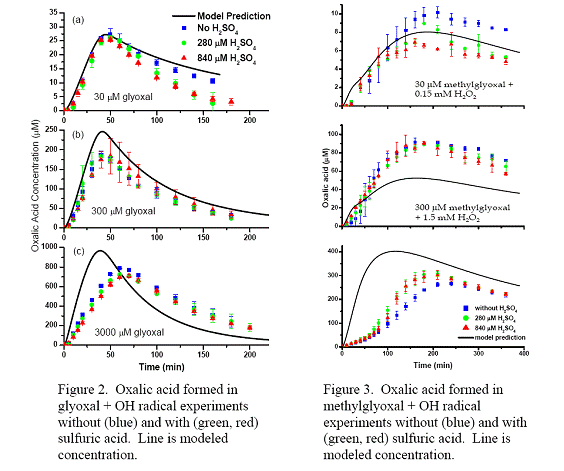
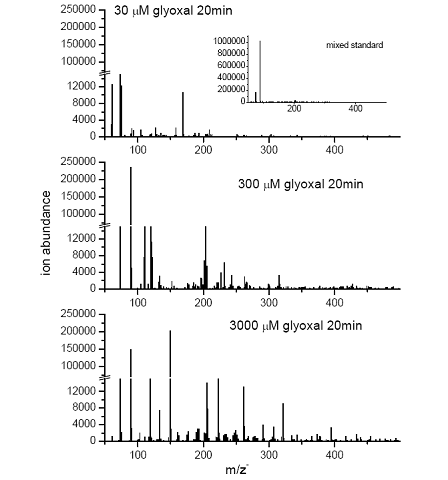

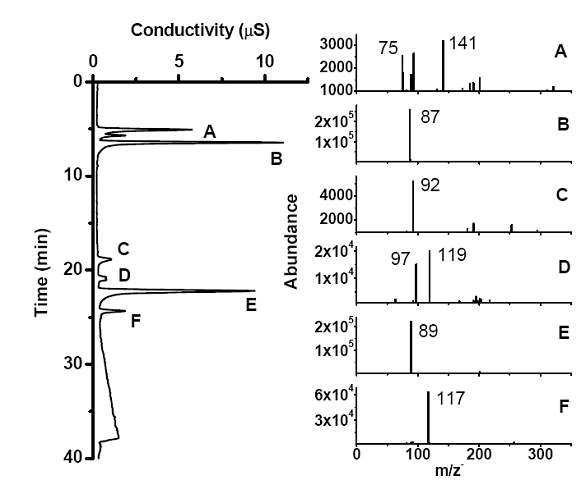
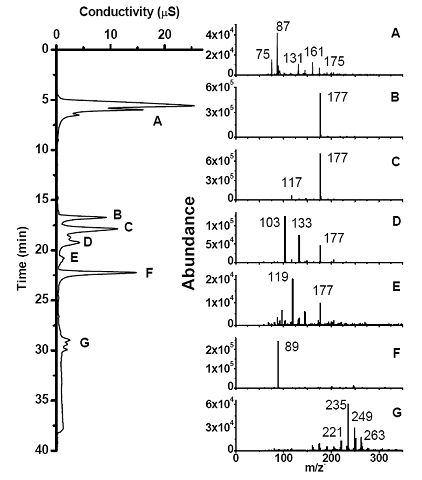


Future Activities:
In the subsequent reporting period we will focus on completing Objective 2. We also will use the model to examine how it can be simplified further for inclusion in CMAQ and other models. We will continue to work with our EPA collaborator, Annmarie Carlton, who is improving the treatment of aqueous chemistry in the CMAQ model. In addition, we will proceed with experiments with and without a nitrogen additive (e.g., nitrate, ammonium, amines).Journal Articles on this Report : 6 Displayed | Download in RIS Format
| Other project views: | All 46 publications | 16 publications in selected types | All 16 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Altieri KE, Seitzinger SP, Carlton AG, Turpin BJ, Klein GC, Marshall AG. Oligomers formed through in-cloud methylglyoxal reactions: chemical composition, properties, and mechanisms investigated by ultra-high resolution FT-ICR mass spectrometry. Atmospheric Environment 2008;42(7):1476-1490. |
R833751 (2008) R833751 (2009) R833751 (2010) R833751 (Final) R831073 (Final) |
Exit Exit Exit |
|
|
Altieri KE, Turpin BJ, Seitzinger SP. Oligomers, organosulfates, and nitrooxy organosulfates in rainwater identified by ultra-high resolution electrospray ionization FT-ICR mass spectrometry. Atmospheric Chemistry and Physics 2009;9(7):2533-2542. |
R833751 (2009) R833751 (Final) |
Exit Exit |
|
|
Altieri KE, Turpin BJ, Seitzinger SP. Composition of dissolved organic nitrogen in continental precipitation investigated by ultra-high resolution FT-ICR mass spectrometry. Environmental Science & Technology 2009;43(18):6950-6955. |
R833751 (2009) R833751 (2010) R833751 (Final) |
Exit Exit Exit |
|
|
Carlton AG, Turpin BJ, Altieri KE, Seitzinger SP, Mathur R, Roselle SJ, Weber RJ. CMAQ model performance enhanced when in-cloud secondary organic aerosol is included:comparisons of organic carbon predictions with measurements. Environmental Science & Technology 2008;42(23):8798-8802. |
R833751 (2008) R833751 (2009) R833751 (2010) R833751 (Final) R831073 (Final) |
Exit Exit Exit |
|
|
Ervens B, Carlton AG, Turpin BJ, Altieri KE, Kreidenweis SM, Feingold G. Secondary organic aerosol yields from cloud-processing of isoprene oxidation products. Geophysical Research Letters 2008;35(2):L02816 (5 pp.). |
R833751 (2008) R833751 (2009) R833751 (2010) R833751 (Final) R831073 (Final) |
Exit Exit |
|
|
Tan Y, Perri MJ, Seitzinger SP, Turpin BJ. Effects of precursor concentration and acidic sulfate in aqueous glyoxal–OH radical oxidation and implications for secondary organic aerosol. Environmental Science & Technology 2009;43(21):8105-8112. |
R833751 (2009) R833751 (2010) R833751 (Final) |
Exit Exit Exit |
Progress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
