Grantee Research Project Results
Final Report: Fundamental Understanding and Performance Enhancement of Conductive Adhesive for Microelectronic Packaging Applications
EPA Grant Number: R831489Title: Fundamental Understanding and Performance Enhancement of Conductive Adhesive for Microelectronic Packaging Applications
Investigators: Wong, C. P.
Institution: Georgia Institute of Technology
EPA Project Officer: Aja, Hayley
Project Period: December 22, 2003 through December 21, 2008
Project Amount: $325,000
RFA: Technology for a Sustainable Environment (2003) RFA Text | Recipients Lists
Research Category: Nanotechnology , Pollution Prevention/Sustainable Development , Sustainable and Healthy Communities
Objective:
A novel flexible electrically conductive adhesive (FECA) has been designed for electronic interconnect applications that require high flexibility on the flexible substrates and connectors. The physical properties of the resulting ECAs such as the elastic modulus, glass transition temperatures, adhesion strength and electrical properties were characterized by differential scanning calorimetry (DSC), dynamic mechanical analyzer (DMA), die shear tester and RCL multimeter. A high performance FECA with tunable modulus, flowability, glass transition temperature, electrical conductivity and adhesion strength was developed for the next-generation large scale flexible flat panel displays and printed electronics applications. Additionally, high transparency and flexibility ECAs have been achieved.
Summary/Accomplishments (Outputs/Outcomes):
Research Highlights
Rongwei Zhang, Jack K. Moon and C.P. Wong (PI)
Polymer-based electrically conductive adhesives (ECAs) have drawn much attention as an environmentally friendly solution for lead-free interconnects due to the advantages of environmental friendliness, mild processing conditions, fewer processing steps, and especially, the fine pitch interconnect capability due to the availability of small-sized conductive fillers. Therefore, ECAs have been widely used in bonding the flex circuit of a glass LCD display to the mating of conducting electrode traces onto a PCB, or attaching a component lead to a matching pad on a thermally-sensitive printed circuit board, etc [1-5].
Conductive adhesives can be categorized into isotropically conductive adhesives (ICA with 2~5 μm conductive fillers) and anisotropically conductive adhesives (ACA with 5~7 μm sized conductive fillers) as shown in Figure 1. Different adhesive types are being adapted as interconnect materials for surface mount technology processes, such as chip on glass (COG), chip on flex (COF) and flip-chip bonding technologies in electronic packaging industries.
Traditionally, rigid epoxy resins are used as polymer matrix for ECA to provide chemical bonding, excellent mechanical and adhesion strengths. However, with the popularity of flex circuits or flexible substrates in particular for large scale flexible flat panel displays, there are some limitations when attaching the traditional ECAs materials to the flex due to poor flexibility of the ECA interconnect, especially under highly mechanical bending conditions of printed electronics. Moreover, the internal stress generated by rigid ECA tends to result in the interconnect failure and affects the contact reliability. If the resulting ECAs can readily release the thermal stress or absorb the stress efficiently, the joint failure due to thermomechanical stresses could be dramatically reduced. In addition, flexible interconnects allow for the highly compact electronic packaging. To achieve flexibility, thin film polyimide or polyurethane has been used. However, the poor adhesion strength and high electrical resistance have restricted their applications in ECAs.
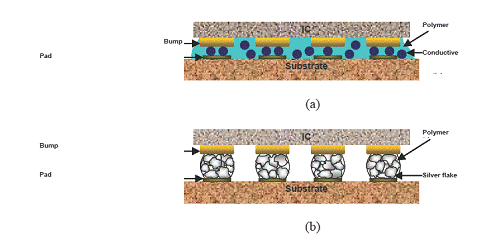
Figure 1. Schematic illustrations of (a) ACA and (b) ICA in flip-chip bonding interconnections.
In addition to flexibility, we aim at developing transparent FECA with good electrical conductivity. Generally, a lower filler loading is required to achieve the transparency in a composite, since most conductive fillers tend to block the light. However, a relatively high loading level is needed for ECA to achieve the electrical conductivity. At low filler concentrations, electrically conductive fillers are dispersed within the polymer matrix. Above certain filler concentrations, conductive particles contact each other and form a three-dimensional network (Figure 2 (a)). This critical concentration is known as a percolation threshold (Vc), at which the resistivity of the composite drops dramatically (Figure 2 (c)). The volume fraction of the conductive filler in an ICA should be equal to or higher than the critical volume fraction to achieve desirable conductivity. In order to achieve the transparent ECA, the Ag-coated fiber was used as a conductive filler. As compared in Figure 2(a), by using the conductive fibers/wires (high aspect ratio fillers), the conductivity of polymer composite can be achieved at a much lower filler loading level (Figure 2 (b)).
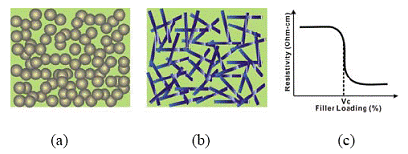
Figure 2. Schematic illustrations of conductivity establishment for polymer composites with (a) spherical fillers, (b) high aspect ratio fillers (fiber/wire) and (c) percolation threshold (Vc) in a resistivity vs. filler loading plot.
In this paper, a flexible epoxy with good adhesion and reasonable shrinkage is introduced into an ECA formulation. A mixture of a flexible epoxy and a typical rigid epoxy is employed as the matrix for flexible ECAs with silver flakes as conductive fillers. Various ratios of epoxies were studied to combine the advantages of flexibility, mechanical strength, adhesion, shrinkage of different epoxies and to optimize the materials properties. By tuning the ratios of epoxies and fillers, the elastic modulus, glass transition temperatures, adhesion strength, electrical conductivity, and impact strength can be modified accordingly to the requirements for different applications. Moreover, with the introduction of the Ag-coated fiber, the transparency of the resulting ECA can be achieved as high as 80 wt% at 550 nm. The resulting transparent and flexible ECA materials have promising applications in the field of emerging printed electronics such as flex display panels and solar cell electrodes.
Experimental
A diglycidyl ether of bisphenol-F (DGEBF) epoxy resin and a flexible epoxy were used as matrix resins. An anhydride type hardener was employed as a crosslinker. Two types of silver flakes were donated by Ferro Co. and incorporated by 80 wt% with 1:1 ratio in the ECA formulations.
The resistivity of the ECAs was calculated from the bulk resistance of the specimen with specific dimensions. Two strips of an adhesive tape were applied onto a pre-cleaned glass slide with a gap of 6.62 mm between these two strips. The conductive adhesive paste was then printed on the glass slide, and then the tapes were removed. After cure, the bulk resistance (R) of this ECA strip was measured as well as the size of the specimen. The bulk resistivity, ρ, was calculated using Equation 1:

where l, w, t are the length, width and thickness of the sample, respectively.
The specimens were exposed to the condition of 85 oC/85% RH (in a temperature and humidity chamber from Lunaire Environmental, model EO932W-4). The contact resistance of each specimen on test coupons was measured periodically during aging.
Dynamic mechanical properties of cured conductive adhesives were investigated using a dynamic mechanical analyzer (DMA) from TA Instruments, model 2980, with a film tension clamp. After a sample was mounted on the clamp, the temperature was raised from 25 oC to 200 oC at a heating rate of 3 oC /min. The sample was studied under an oscillation mode with a frequency of 1 Hz. Tan delta versus temperature was recorded.
For adhesion study, a die shear tester (Dage series 4000) was utilized, with a configuration as shown in Figure 2. The size of the silicon die was 2 mm by 2 mm.
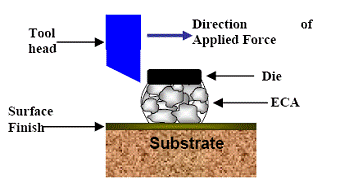
Figure 2. Schematic illustration of die shear test.
Results and Discussion
Mechanical Properties of FECAs
The flexible epoxy was mixed with the typical rigid epoxy with various ratios before adding the silver flake fillers. The storage moduli with temperature of the mixtures of flexible and rigid ECAs are shown in Figure 3 (a). With increasing the flexible epoxy concentration, the storage modulus of the resulting ECA at room temperature decreases from 5000 MPa to 1000 MPa (see Figure 3 (b)), indicating a more flexible structure. The glass transition temperature (Tg) of the FECA decreased obviously with increasing the amount of the flexible epoxy in the polymer resin, as shown in Figure 4. Moreover, only a single Tg in the measured temperature range was observed for each formulation, indicating that the resulting ECA was a single homogeneous phase. Furthermore, the loss modulus at room temperature could also be tuned from 100 MPa to 450 MPa with increasing the ratio of flexible to rigid epoxy resins, as illustrated in Figure 5.
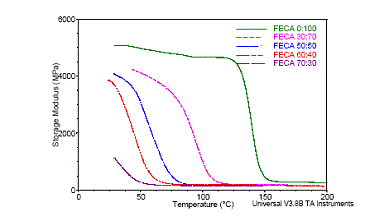
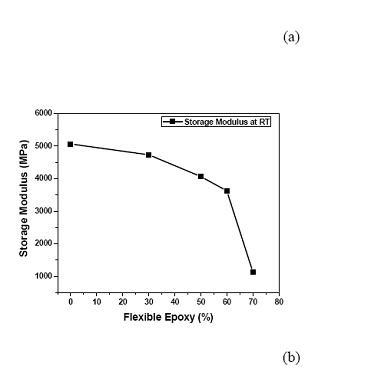
Figure 3(a). Changes of storage moduli of ECAs with different ratios of flexible epoxy to rigid epoxy; Figure 3(b). Storage moduli of ECAs with different ratios of flexible epoxy to rigid epoxy at room temperature.
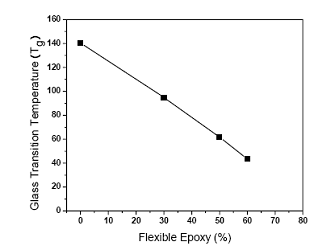
Figure 4. Glass transition temperatures (Tgs) of FECA with different ratios of flexible epoxy to rigid epoxy
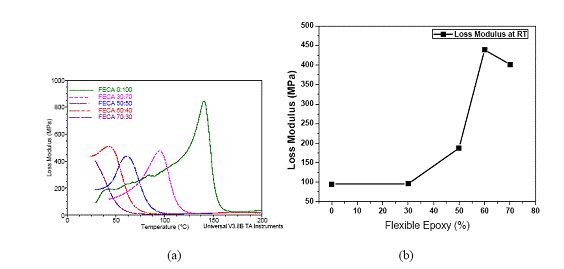
Figure 5(a). Changes of loss moduli of ECAs with different ratios of flexible epoxy to rigid epoxy; (b) Loss moduli of ECAs with different ratios of flexible epoxy to rigid epoxy at room temperature.
Electrical conductivity and reliability test of FECA
Generally, an ECA has low conductivity before cure, but the conductivity increases dramatically after the ECA is cured, provided the filler loading exceeds the percolation threshold of the composite. The ECA achieves conductivity during the cure process, mainly through more intimate contacts between Ag flakes caused by cure shrinkage of the polymer binder. As previous research work in our group has demonstrated that resin cure shrinkage play a very important role in conductivity establishment of a conductive adhesive [6, 7]. It has been discovered that an ICA with higher cure shrinkage generally shows better conductivity. However, for flexible ECAs, it was found that with increasing the amount of the flexible epoxy in the ECA formulations, there was less shrinkage after cure while the electrical conductivity was higher with more flexible ECA. The exact reason is under investigation.
Figure 6 shows the changes of bulk resistivity and contact resistance of a series of ECAs with different content of flexible epoxy during 85 oC/85% RH aging. All the ECA formulations show relatively stable bulk resistivity (Figure 6 (a)) and contact resistance (Figure 6 (b)) after aging around 500 hrs. It is noted that the bulk resistivity decreased in the first 24 hrs may be due to the post curing of epoxy resins (see Figure 6 (a)).
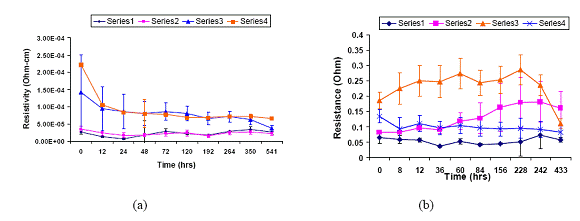
Figure 6. The mean bulk resistivity (a) and mean contact resistance (b) on Au surfaces with different ratios of flexible epoxy to rigid epoxy during 85 oC/85% RH aging (error bar: 2 times standard deviation).
Adhesion strength
FECAs with different ratios of flexible epoxy and rigid epoxy were formulated and the die shear strength of the FECAs on glass, polyimide and gold substrate were measured, respectively. Previously, we have reported that the adhesion strength was generally reduced with the addition of flexible epoxy. The continuous decrease of the adhesion strength was observed for FECAs on a glass substrate. However, for polyimide and gold substrates, no significant decrease in the adhesion strength was observed for the ECA with up to 50 wt% flexible epoxy. Comparing the adhesion strength of FECAs on various substrates, the adhesion strength of FECAs on Au was the lowest with the same resin formulation.
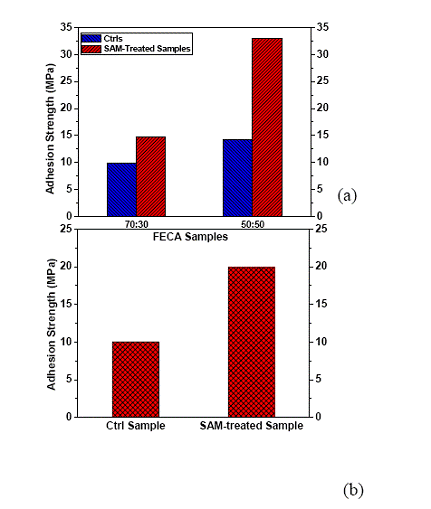
Figure 7. Adhesion strength of FECA on Au (a) and Cu (b) surfaces with SAMs.
High adhesion strength is a critical parameter in fine pitch interconnection that is fragile to shocks encountered during assembly, handling and lifetime. In order to enhance the adhesion strength of FECAs on a Au surface, a di-functional self-assembled monolayer (SAM) was used to treat the Au surface before the die shear test. Our previous study has demonstrated that SAMs with various functional groups tend to have strong affinity on specific metal surfaces and form the strong connection with the metal particles or surfaces [8-10]. In particular, conjugated molecules have a smaller band gaps between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) and possess delocalized conjugated π-electrons that can contribute to conduction. In semiconductors, self-assembled molecular wires have seen importance in tuning the metal work function (Φ) and electrical conduction of metal-molecule contact [11-13].
The adhesion strengths of FECA on Au and Cu surfaces with di-functional conjugated SAM treatment were measured for the formulation of 50wt% and 70wt% flexible epoxy, respectively. As shown in Figure 7, with the SAM treatment, the adhesion strength of FECA on a Au substrate was significantly enhanced. Typically, for the di-functional SAM molecule used, one functional group could strongly adhere to the Au surface, while the other end of the SAM molecule attached to the epoxy. As such, a strong bridge/coupling was formed between the FECA and the Au substrate and increased the adhesion strength. The molecular wire’s function is similar to a chemical coupling agent, but due to the possible formation of its highly ordered layer, the surface coverage of the SAM molecule is much higher than that of coupling agents. Consequently, a much higher adhesion strength could be achieved. Furthermore, typical coupling agents such as silanes may sacrifice the electrical conductivity of FECA. The short chain di-functional SAM molecules, usually can further improve the electrical conductivity, as reported by the previous study [14].
Transparency
In order to develop transparent and flexible ECAs, high aspect ratio conductive fillers should be employed, since the percolation can be achieved at a much lower volume fraction in a high aspect ratio filler system than in low aspect ratio one. The lower filler loading will render the transparency as well as flexibility of the ECA without significantly adverse effects on the properties. By incorporating the micron meter-sized Ag-coated glass fiber, our preliminary results show that conductivity was achieved at much lower filler loading with Ag-coated glass fibers and transparency at visible range (transmission at 550 nm (80%)) was achieved (Figure 8). The key issues for the preparation of transparent and flexible ECAs are: (1) the proper dispersion of conductive fillers and prevent the unfavorable aggregation of conductive filler during curing. From this point of view, flexible epoxy with silver nanowires (as shown in Figure 10) may have advantages to enhance the dispersion. However, detail study is under investigation at present.
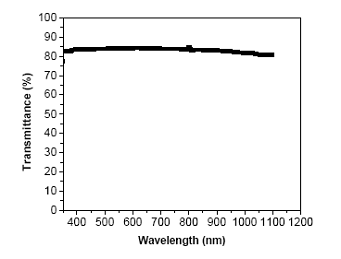
Figure 8. Light transmittance of 12 μm flexible ECA with 30 wt% Ag-coated glass fibers (80 % transmission at 550 nm).

Figure 9. Transmission electron micrograph of silver nanowires synthesized in our lab.
Conclusions:
A variety of FECAs have been formulated to provide a wide range of electrical and mechanical properties. By tuning the ratios of flexible and rigid epoxies, the electrical and mechanical properties can be modified accordingly to satisfy requirements for different applications. In particular, good flexibility, high electrical conductivity, and good reliability could be achieved. Adhesion strength of FECA can be significantly enhanced with treatment of di-functional SAM molecules. Additionally, high transparent and flexible ECA with reasonable conductivity has been achieved. The transparent and flexible ECAs developed will have a promising application in large scale flexible printed electronics such as flat panel displays, solar cells, etc.
Acknowledgments
The authors acknowledge the Environmental Protection Agency EPA) for financial support.
References:
1. Y. Li, K. S. Moon, and C. P. Wong, “Electronics without lead,” Science, vol. 308 (2005) pp. 1419-1420.
2. Y. Li and C. P. Wong, “Recent advances of conductive adhesives as a lead-free alternative in electronic packaging: materials, processing, reliability and applications”, Materials Science & Engineering, R: Reports, vol. R51 (2006) pp. 1-35.
3. J. S. Hwang, (Ed.) “In Environment-Friendly Electronics: Lead-free Technology”, Electrochemical Publications Ltd., Port Erin, UK, 2001, Chapter 1, pp. 4-10.
4. J. Lau, C. P. Wong, N. C. Lee, S. W. R. Lee, “In Electronics Manufacturing: with Lead-Free, Halogen-Free, and Conductive-Adhesive Materials”, McGraw Hill, New York, NY, 2002.
5. J. Liu, (Ed.) “Conductive adhesives for Electronics Packaging”, Electrochemical Publications Ltd. Isle of Man, British Isles, 1999, Chapter 1.
6. D. Lu and C. P. Wong, “Effects of shrinkage on conductivity of isotropic conductive adhesives”, Int. J. Adhes. Adhes., vol. 20 (2000) pp. 189-193.
7. D. Lu, Q. K. Tong, and C. P. Wong, “Conductivity mechanisms of isotropic conductive adhesives (ICA's)”, IEEE Trans. on Electron. Packag. Manufact., vol. 22 (1999) pp. 223-227.
8. Y. Li, K. Moon and C. P. Wong, “Adherence of Self-Assembled Monolayers on Gold and their Effects for High Performance Anisotropic Conductive Adhesives”, J. Electron. Mater., vol. 34 (2005) pp. 266-271.
9. Y. Li, K. Moon, and C. P. Wong, “Monolayer Protected Silver Nano-particle Based Anisotropic Conductive Adhesives (ACA): Electrical and Thermal Properties Enhancement”, J. Electron. Mater., vol. 34 (2005) pp. 1573-1578.
10. K. S. Moon, C. Rockett and C.P. Wong, “Adhesion improvement of thermoplastic-based isotropic conductive adhesives under humid environment using self-assembled monolayer compounds”, J. Adh. Sci. & Technol., vol 18 (2004) pp. 153~167.
11. B. de Boer, A. Hadipour, M. M. Mandoc, T. van Woudenbergh, P. W. M. Blom, “Tuning of Metal Work Functions with Self-Assembled Monolayers”, Adv. Mater. vol. 17 (2005) pp. 621-625.
12. C. Joachim, J. K. Gimzewski, A. Aviram, “Electronics using hybrid-molecular and mono-molecular devices” Nature 408 (2000) pp. 541-548.
13. Q. Sun, A. Selloni, G. Scoles, “Electronic Structure of Metal/Molecule//Metal Junctions: a Density Functional Theory Study of the Influence of the Molecular Terminal Group”, J. Phys. Chem. B, vol. 110 (2006) pp. 3493-3498.
14. Y. Li, K. Moon and C. P. Wong, “Electrical Property Improvement of Electrically Conductive Adhesives through in- situ Replacement by Short-Chain Difunctional Acids”, IEEE Trans. Compon. Packag. Technol., Vol 29 (2006) pp. 173-178.
Journal Articles on this Report : 27 Displayed | Download in RIS Format
| Other project views: | All 81 publications | 33 publications in selected types | All 32 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Chiang H-W, Chung C-L, Chen L-C, Li Y, Wong CP, Fu S-L. Processing and shape effects on silver paste electrically conductive adhesives (ECAs). Journal of Adhesion Science and Technology 2005;19(7):565-578. |
R831489 (2005) R831489 (Final) |
Exit |
|
|
Dong H, Moon K-S, Wong CP. Molecular dynamics study on the coalescence of Cu nanoparticles and their deposition on the Cu substrate. Journal of Electronic Materials 2004;33(11):1326-1330. |
R831489 (2004) R831489 (Final) |
Exit Exit |
|
|
Dong H, Zhang Z, Wong CP. Molecular dynamics study of a nano-particle joint for potential lead-free anisotropic conductive adhesives applications. Journal of Adhesion Science and Technology 2005;19(2):87-94. |
R831489 (2004) R831489 (2005) R831489 (Final) |
Exit |
|
|
Dong H, Fan L, Moon K-S, Wong CP, Baskes MI. MEAM molecular dynamics study of lead free solder for electronic packaging applications. Modelling and Simulation in Materials Science and Engineering 2005;13(8):1279. |
R831489 (Final) |
Exit Exit |
|
|
Dong H, Moon K-S, Wong CP. Molecular dynamics study of nanosilver particles for low-temperature lead-free interconnect applications. Journal of Electronic Materials 2005;34(1):40-45. |
R831489 (2004) R831489 (2005) R831489 (Final) |
Exit Exit |
|
|
Dong H, Li Y, Yim MJ, Moon KS, Wong CP. Investigation of electrical contact resistance for nonconductive film functionalized with π-conjugated self-assembled molecules. Applied Physics Letters 2007;90(9):092102 (3 pp.). |
R831489 (2006) R831489 (2007) R831489 (Final) |
Exit Exit |
|
|
Jiang HJ, Moon K-S, Zhang ZQ, Pothukuchi S, Wong CP. Variable frequency microwave synthesis of silver nanoparticles. Journal of Nanoparticle Research 2006;8(1):117-124. |
R831489 (2004) R831489 (2005) R831489 (Final) |
Exit Exit |
|
|
Jiang H, Moon K-S, Lu J, Wong CP. Conductivity enhancement of nano silver-filled conductive adhesives by particle surface functionalization. Journal of Electronic Materials 2005;34(11):1432-1439. |
R831489 (2005) R831489 (Final) |
Exit Exit |
|
|
Jiang H, Moon K-S, Li Y, Wong CP. Surface functionalized silver nanoparticles for ultrahigh conductive polymer composites. Chemistry of Materials 2006;18(13):2969-2973. |
R831489 (2006) R831489 (Final) |
Exit Exit Exit |
|
|
Jiang H, Zhu L, Moon K-S, Wong CP. Low temperature carbon nanotube film transfer via conductive polymer composites. Nanotechnology 2007;18(12):125203 (4 pp.). |
R831489 (Final) |
Exit Exit |
|
|
Jiang H, Moon K-S, Sun Y, Wong CP, Hua F, Pal T, Pal A. Tin/indium nanobundle formation from aggregation or growth of nanoparticles. Journal of Naonoparticle Research 2008;10(1):41-46. |
R831489 (Final) |
Exit Exit Exit |
|
|
Li Y, Xiao F, Moon K-S, Wong CP. Novel curing agent for lead-free electronics: amino acid. Journal of Polymer Science Part A: Polymer Chemistry 2006;44(2):1020-1027. |
R831489 (2005) R831489 (2006) R831489 (Final) |
Exit |
|
|
Li Y, Moon K-S, Wong CP. Materials science. Electronics without lead. Science 2005;308(5727):1419-1420. |
R831489 (2005) R831489 (Final) |
Exit |
|
|
Li Y, Moon K-S, Wong CP. Adherence of self-assembled monolayers on gold and their effects for high-performance anisotropic conductive adhesives. Journal of Electronic Materials 2005;34(3):266-271. |
R831489 (2005) R831489 (Final) |
Exit Exit |
|
|
Li Y, Moon K-S, Wong CP. Monolayer-protected silver nano-particle-based anisotropic conductive adhesives:electrical and thermal properties. Journal of Electronic Materials 2005;34(12):1573-1578. |
R831489 (2005) R831489 (Final) |
Exit Exit |
|
|
Li Y, Moon K-S, Wong CP. Reliability improvement of conductive adhesives on tin (Sn) surfaces. Journal of Adhesion Science and Technology 2005;19(16):1427-1444. |
R831489 (2005) R831489 (Final) |
Exit |
|
|
Li Y, Moon K-S, Whitman A, Wong CP. Enhancement of electrical properties of electrically conductive adhesives (ECAs) by using novel aldehydes. IEEE Transactions on Components and Packaging Technologies 2006;29(4):758-763. |
R831489 (2006) R831489 (Final) |
Exit |
|
|
Li Y, Moon K-S, Wong CP. Enhancement of electrical properties of anisotropically conductive adhesive joints via low temperature sintering. Journal of Applied Polymer Science 2006;99(4):1665-1673. |
R831489 (2005) R831489 (2006) R831489 (Final) |
Exit |
|
|
Li Y, Wong CP. Recent advances of conductive adhesives as a lead-free alternative in electronic packaging: materials, processing, reliability and applications. Materials Science and Engineering R: Reports 2006;51(1-3):1-35. |
R831489 (2005) R831489 (2006) R831489 (Final) |
Exit |
|
|
Li Y, Moon K-S, Wong CP. Electrical property improvement of electrically conductive adhesives through in-situ replacement by short-chain difunctional acids. IEEE Transactions on Components and Packaging Technologies 2006;29(1):173-178. |
R831489 (2005) R831489 (2006) R831489 (Final) |
Exit |
|
|
Li Y, Wong CP. Monolayer protection for eletrochemical migration control in silver nanocomposite. Applied Physics Letters 2006;89(11):112112. |
R831489 (2006) R831489 (Final) |
Exit Exit |
|
|
Li Y, Wong CP. High performance anisotropic conductive adhesives for lead-free interconnects. Soldering & Surface Mount Technology 2006;18(2)33-39. |
R831489 (2006) R831489 (Final) |
Exit |
|
|
Li Y, Xiao F, Wong CP. Novel, environmentally friendly crosslinking system of an epoxy using an amino acid: tryptophan-cured diglycidyl ether of bisphenol A epoxy. Journal of Polymer Science Part A: Polymer Chemistry 2007;45(2):181-190. |
R831489 (2006) R831489 (2007) R831489 (Final) |
Exit |
|
|
Li Y, Yim MJ, Moon KS, Wong CP. Novel nano-scale conductive films with enhanced electrical performance and reliability for high performance fine pitch interconnect. IEEE Transactions on Advanced Packaging 2009;32(1):123-129. |
R831489 (Final) |
Exit |
|
|
Moon K-S, Li Y, Xu JW, Wong CP. Lead-free interconnect technique by using variable frequency microwave. Journal of Electronic Materials 2005;34(7):1081-1088. |
R831489 (2004) R831489 (2005) R831489 (Final) |
Exit Exit |
|
|
Moon K-S, Dong H, Maric R, Pothukuchi S, Hunt A, Li Y, Wong CP. Thermal behavior of silver nanoparticles for low-temperature interconnect applications. Journal of Electronic Materials 2005;34(2):168-175. |
R831489 (2005) R831489 (Final) |
Exit Exit |
|
|
Yim MJ, Li Y, Moon K-S, Paik KW, Wong CP. Review of recent advances in electrically conductive adhesive materials and technologies in electronic packaging. Journal of Adhesion Science and Technology 2008;22(14):1593-1630. |
R831489 (Final) |
Exit |
Supplemental Keywords:
Sustainable Industry/Business, RFA, Scientific Discipline, TREATMENT/CONTROL, INTERNATIONAL COOPERATION, POLLUTION PREVENTION, Technology for Sustainable Environment, Chemical Engineering, Sustainable Environment, Environmental Chemistry, Economics and Business, Technology, Energy, environmentally conscious manufacturing, lead reduction, energy conservation, electrically conductive adhesive, corrosion resistant, electronic packaging, clean technologies, cleaner production, energy efficiency, waste reductionProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2007 Progress Report
- 2006 Progress Report
- 2005 Progress Report
- 2004 Progress Report
- Original Abstract
32 journal articles for this project
