Grantee Research Project Results
2023 Progress Report: Practical PFAS Treatment with Functionalized Sawdust
EPA Grant Number: SV840386Title: Practical PFAS Treatment with Functionalized Sawdust
Investigators: Tu, Maobing , Lu, Mingming , Harrison, Canaan , Udeshi Edirisinghe, Don Isanka
Institution: University of Cincinnati
EPA Project Officer: Aja, Hayley
Phase: II
Project Period: April 1, 2022 through March 31, 2024 (Extended to March 31, 2025)
Project Period Covered by this Report: April 1, 2023 through March 31,2024
Project Amount: $99,895
RFA: P3 Awards: A National Student Design Competition for Sustainability Focusing on People, Prosperity and the Planet - Phase 2 (2022) Recipients Lists
Research Category: P3 Awards , P3 Challenge Area - Safe and Sustainable Water Resources
Objective:
EPA designated PFOA and PFOS as hazardous substances and issued drinking water standards to protect people from exposure to harmful PFAS in 2024. The objective of this Phase II research is to develop and demonstrate PFAS removal from wastewater and drinking water using biomass-based functionalized sawdust (FS). The project aims to reduce the environmental and human health impact of PFAS. Significant progress has been made by undergraduate and graduate students, keeping the project on schedule and meeting milestones.
In the past year, three objectives have been achieved: 1. Determining the effect of PFAS chain length and functional group on PFAS removal with FS. 2. Developing a pilot-scale test for PFAS removal from wastewater with FS and GAC. 3. Integrating combustion and mineralization for spent FS.
One more objective will be pursued in the subsequent project period: 4. Performing techno-economic analysis (TEA) and life cycle assessment (LCA) of PFAS removal from wastewater with FS. The project will provide training opportunities for 4-8 undergraduate students and 2-3 graduate students.
Progress Summary:
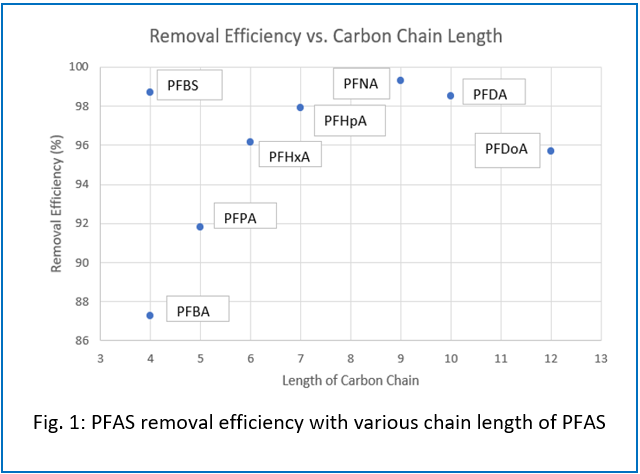
In this Phase II project, eight PFAS compounds with various chain length and functional groups were tested for their removal efficiency with FS at room temperature for 48 hours. Langmuir and Freundlich isotherms were used to characterize adsorption capacity. It was observed that FS showed promising removal efficiency for all eight PFAS compounds (Fig. 1). The chain length significantly influenced PFAS removal efficiency with FS. For example, PFBA, with a shorter chain, exhibited a lower removal efficiency of 87%, while PFBS, despite having the same chain length as PFBA, achieved a higher removal efficiency. This discrepancy could be attributed to PFBS’s more electronegative sulfonate group compared to the less electronegative carboxyl group of PFBA. Langmuir adsorption isotherms revealed that FS had the highest adsorption capacity 163.93 mg/g for PFHpA and the lowest 7.98 mg/g for PFDoA, adsorption capacity increased with chain length, peaking at PFHpA (7 carbons) before decreasing with longer chains. This study suggests that both chain length and functional groups in PFAS significantly impact PFAS removal efficiency with FS.
A column test was conducted as a small pilot-scale evaluation of FS for PFAS removal from wastewater or drinking water. Students calculated the empty bed contact time (EBCT) around 2 hours based on the volume of absorbent and flow rate. Based on EBCT, the samples were collected every 30 minutes over a total run time of 8.5 hours. The column assembly and piston pump setup were successfully tested with PFDA on FS, demonstrating that activated sawdust absorbed all the PFAS through the first four EBCTs. At 8 hours, low levels of PFAS were detected in treated water samples. The adsorption column showed >99% removal efficiency for PFDA after 8.5 hour (with initial PFDA at 100 mg/L) [Fig.1]. The results indicate that adsorption column with FS is potentially feasible solution for PFAS removal from wastewater or drinking water.
The spent FS with adsorbed PFAS must be treated before disposal. Combustion of PFAS laden FS is a promising approach to address the disposure issue. Preliminary combustion experiments with PFDoA, PFBA, PFBS, and PFHpA (45 mg each) were conducted, and recovery analysis for each compound was performed. Spent sawdust samples were combusted, and the system was analyzed for inorganic, mineralized fluorine byproducts. Pyrolysis at 1000ºC was performed to fully convert organic byproducts to mineralized fluorine. To determine how much PFAS was converted to mineralized fluorine, fluoride recoveries were quantified for each compound using ion chromatograph (IC). PFHpA and PFDA achieved higher recoveries of approximately 74.7% and 70.4%, respectively, while recoveries for other compounds were around 50%. Preliminary combustion demonstrated effective fluorine recovery, suggesting that that adsorbed PFAS on FS can be effectively destroyed. The biomass-based FS presents a potential alternative material for water utility companies to address PFAS removal. It aligns well with the EPA mission on water sustainability.
Future Activities:
The planned activity and tasks are on the right track. The detailed future activities will include:
- Repeat PFAS adsorption column test with various PFAS compounds and conditions.
- Continue the experiment of combustion and mineralization of spent FS and determine the major combustion products.
- Conduct TEA and LCA analysis of PFAS removal from wastewater with FS.
Journal Articles:
No journal articles submitted with this report: View all 2 publications for this projectSupplemental Keywords:
PFAS, functionalized sawdust, chain length, treatment, column test, thermal combustion and mineralization, wastewater
Progress and Final Reports:
Original AbstractP3 Phase I:
Practical PFAS Treatment with Sawdust | 2020 Progress Report | Final ReportThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.