Grantee Research Project Results
2020 Progress Report: Reducing the reliance on early-life stage testing with relevance to euryhaline fishes: Development and implementation of in-vitro assays predictive of early life stage toxicity and population-level effects in Menidia beryllina
EPA Grant Number: R839503Title: Reducing the reliance on early-life stage testing with relevance to euryhaline fishes: Development and implementation of in-vitro assays predictive of early life stage toxicity and population-level effects in Menidia beryllina
Investigators: Brander, Susanne M , Chappell, Patrick , Armbrust, Kevin , White, Wilson
Institution: Oregon State University , Louisiana State University
EPA Project Officer: Aja, Hayley
Project Period: August 1, 2019 through April 23, 2025
Project Period Covered by this Report: August 1, 2019 through July 31,2020
Project Amount: $849,988
RFA: Advancing Actionable Alternatives to Vertebrate Animal Testing for Chemical Safety Assessment (2018) RFA Text | Recipients Lists
Research Category: Chemical Safety for Sustainability
Objective:
Over the past decade great strides have been made in minimizing the use of vertebrates for toxicity testing focused on human health outcomes. However, although numerous approaches and models that facilitate the reduction of vertebrates in human toxicity testing currently exist, far fewer alternatives are available for use in ecotoxicological applications, particularly for marine and estuarine ecosystems. For example, the current marine and estuarine species approved for EPA Whole Effluent Toxicity (WET) testing are Cyprinodon variegatus (Sheepshead minnow) and Menidia beryllina (Inland silverside). Neither species has appropriate in vitro alternatives available to limit the use of live animals for regulatory and research purposes. Assessing ecotoxicological effects in estuarine systems is crucial, given the importance and value of those ecosystems (and their vulnerability to pollution. Further complicating the assessment of toxicity in estuarine systems is the altered behavior of chemicals in seawater, which influences water solubility and concomitant bioavailability and thus raises the potential for differential responses to chemicals dependent on salinity, not to mention the likelihood of differences in osmoregulatory strategies influencing the uptake and metabolism of chemicals between fishes inhabiting fresh and saline waters. For example, fish in saline waters drink constantly to remain isotonic with their surroundings, while freshwater fish urinate frequently to achieve the same goal. Regardless of these challenges, a marine model that uses cell lines to reduce reliance on live fish for the purposes of identifying, prioritizing, and evaluating potential toxicants is greatly needed. Additionally, the development of dosimetry that accurately reflects bioavailability across a salinity gradient would refine this testing, allowing cell lines to be used at a minimum as a first-tier approach to limit the number of toxicants that necessitating in vivo testing.
Experimental Approach: We are developing multiple novel in vitro Menidia assays from tissue types (cardiovascular, hepatic, bone) with demonstrated sensitivity in early life across fish species that are complementary to commonly used fish early life stage and larval growth and survival tests. We also detail plans for the generation of dosimetry for mimicking chemical (pesticide) exposures across a salinity gradient in vitro, and the use of genomic tools (RNA seq, high-resolution bioluminescence gene expression monitoring) and demographic modeling in a “middle-out” approach linking in vivo phenotypic anchors (e.g. developmental deformities, altered growth) to biomarker candidates and population-level outcomes. Ultimately this approach would be informative of existing adverse outcome pathways (AOPs) and may also generate novel AOPs. For the purposes of validating our euryhaline in vivo – in vitro linked model, we plan to conduct exposures with a suite of pesticides from different chemical classes, previously established to be cardiotoxic, hepatotoxic, and osteotoxic in the zebrafish model, and all commonly used in the control of rodents, insects, weeds, or fungus.
Anticipated Results: The major accomplishment of this work will be the creation and validation of a euryhaline in vivo-in vitro linked model with a proposed focus on pesticides already known to affect development in embryonic and larval life stages in freshwater fish, yet with a dearth of information on responses in euryhaline fishes. Ultimately, the approaches developed herein would not only reduce in the number of live fish needed for such testing and improve the efficiency of testing for pesticide registration, but would also reduce the cost and refine the accuracy of risk assessment for other classes of chemicals. This is of mounting importance given the number of new compounds on the market and limited resources available for toxicity testing. We expect to find that most compounds are more toxic at higher salinities, based on results from in vitro and in vivo assays and due to a combination of salting-out of solution and hence becoming more bioavailable, or due differences in metabolism and osmo-regulation.
Progress Summary:
In Vivo exposures - PhD student Sara Hutton and lab technician Emily Pedersen conducted range finding exposures on the Inland Silverside (Menidia beryllina) from January through May of 2020 with only a slight disruption due to COVID-19. Range finding exposures were conducted at 5 and 15 ppt for myclobutanil, paraquat di-chloride, bifenthrin, chlorpyrifos, penconazole, triadimefon, and dicloran. Exposure concentrations are shown in Table 1. Warfarin was not included in range finding studies due to difficulties with solubilizing the compound at the required concentration to induce lethality. LC10 values derived from this study will be used to inform future sub-lethal exposures.
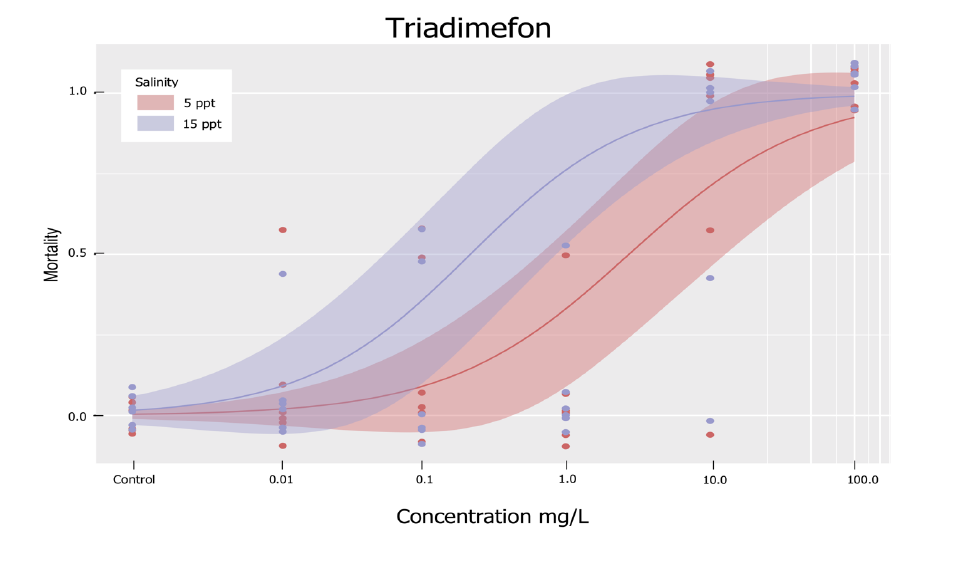
Figure 1. Dose-response curve for Tradimefon, similar curves will included in publication
submitted to Toxics across all biocides tested.
Statistical analysis: LC50 and LC10 values were determined by fitting four parameter log-logistic dose-response curves to the data. LC50 and LC10 values are shown in Table 2. To determine if the differences between the two salinities for any one compound were significant the difference in LC50 and LC10 values between the 5 and 15ppt exposures were obtained, the data was then re-sampled 5000 times in a Monte Carlo Simulation (under the advisement of co-PI White) to determine if the differences were real or random. Results from the analysis revealed that the differences between the LC50 and LC10 values for myclobutanil (p-value = 0.0112), triadimefon (p-value = 0.0024), dicloran (p-value = 0.0284), and paraquat di-chloride (p-value = 0.0456) were statistically significant, while bifenthrin (p-value = 0.6732), chlorpyrifos (p-value = 0.1088), and penconazole (p-value = 0.1104 ) were not. A p-value of less than 0.5 was considered significant.
| Compound | Units | Conc. 1 | Conc. 2 | Conc. 3 | Conc. 4 | Conc. 5 |
| Dicloran | mg/L | 0.0009 | 0.009 | 0.09 | 0.9 | 9.0 |
| Paraquat | mg/L | 0.067 | 0.67 | 6.7 | 67.0 | 670.0 |
| Chlorpyrifos | mg/L | 0.00000096 | 0.0000096 | 0.000096 | 0.00096 | 0.0096 |
| Bifenthrin | mg/L | 0.00002 | 0.0002 | 0.002 | 0.02 | 0.2 |
| Myclobutanil | mg/L | 0.004 | 0.04 | 0.4 | 4.0 | 40.0 |
| Penconazole | mg/L | 0.00107 | 0.0107 | 0.107 | 1.07 | 10.7 |
| Triadimefon | mg/L | 0.01 | 0.1 | 1.0 | 10.0 | 100.0 |
| Chemical | Units | Salinity | LC50 | LC10 |
| Myclobutanil | mg/L | 5 ppt | 4.22* | 0.00577 |
| Myclobutanil | mg/L | 15 ppt | 1.32* | 0.00159 |
| Paraquat | mg/L | 5 ppt | 37.25* | 0.0546 |
| Paraquat | mg/L | 15 ppt | 18.13* | 0.0245 |
| Triadimefon | mg/L | 5 ppt | 2.73** | 0.115 |
| Triadimefon | mg/L | 15 ppt | 0.215** | 0.0115 |
| Penconazole | mg/L | 5 ppt | 0.107* | 0.000235 |
| Penconazole | mg/L | 15 ppt | 0.0202* | 0.0000244 |
| Dicloran | mg/L | 5 ppt | 0.00617 | 0.000288 |
| Dicloran | mg/L | 15 ppt | 0.00224 | 0.0000704 |
| Chlorpyrifos | μg/L | 5 ppt | 8.02394 | 0.13762 |
| Chlorpyrifos | μg/L | 15 ppt | 2.40067 | 0.039305 |
| Bifenthrin | μg/L | 5 ppt | 0.1603 | 0.022723 |
| Bifenthrin | μg/L | 15 ppt | 0.119915 | 0.00533 |
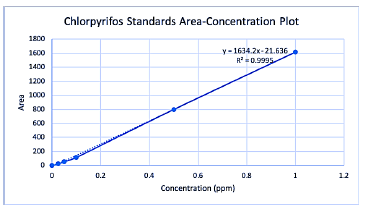
Analytical chemistry: In short, of all the water samples analyzed to date, and penconazole and myclobutanil were detected closest to their nominal values, while all other water samples were detected below their nominal values. Due to the fact that all of these samples were stored frozen for a significant amount of time prior to analysis, due to Covid 19 delays, a freezer suitability study has been started to help gauge how much may be degrading during storage. Furthermore, as indicated by the results, chlorpyrifos is exhibiting substantial hydrolysis to its metabolite 3,5,6-trichloro-2-pyridinol. Results indicate that the 5 ppb chlorpyrifos sample had hydrolyzed by approximately by 80%. Research is being done in our lab to assess the effects of salinity on its hydrolysis rate. Note that the chlorpyrifos sample reported on the attached table was magnified using a liquid liquid extraction (LLE), which was found to have loss up to 50%. Solid Phase Extraction is being utilized for the remainder of the samples which gives approximately 70% recovery. Regarding the egg extraction procedure, ethyl acetate and hexane seem to be extracting some of these compounds from the egg matrix fairly well. Examples are provided in Table 3. More testing is underway to confirm that these solvents work well for the other compounds.
Lastly, we are still determining the exact detection limits of each compound for the GC/MS, triadimefon and myclobutanil can be detected at 2.5 ppb, chlorpyrifos, penconazole, and dicloran can be detected at 5 ppb, and bifenthrin can be detected at 1 ppb. Again, these are not detection limits, these are just the lowest concentrations we have run so far and was able to get results. The focus throughout the coming months will be to assess what the specific detection limits are for each compound on GC/MS as well as analyze the remainder of your water samples.
| Chlorpyrifos Eggs Spiked to 1.17 PPM | Triadimefon Eggs Spiked to 0.033 PPM | ||
| Methanol Extraction | 100% Ethyl Acetate Extraction (A) | ||
| Area | Concentration (ppm) | Area | Concentration (ppm) |
| 180.477 | 0.123677029 | 30.956 | 0.011855911 |
| Hexane Extraction | 80% Ethyl Acetate Hex Extraction (B) | ||
| Area | Concentration (ppm) | Area | Concentration (ppm) |
| 1044 | 0.6520842 | 72.206 | 0.025488218 |
| Ethyl Acetate Extraction | 90% Ethyl Acetate Hex Extraction | ||
| Area | Concentration (ppm) | Area | Concentration (ppm) |
| 1752 | 1.085323706 | 58.811 | 0.021061436 |
| Standards | 70% Ethyl Acetate (B) | ||
| Area | Concentration (ppm) | Area | Concentration (ppm) |
| 0 | 0 | 54.37 | 0.019593774 |
| 23.895 | 0.025 | 70% Ethyl Acetate (A) | |
| 55.057 | 0.05 | Area | Concentration (ppm) |
| 116.668 | 0.1 | 44.747 | 0.016413563 |
| 797.787 | 0.5 | 100% Ethyl Acetate (B) | |
| 1614 | 1 | Area | Concentration (ppm) |
| Slope | Y-Intercept | Standards | |
| -1634.2 | -21.636 | Area | Concentration (ppm) |
| 42.368 | 0.01567351 | ||
| Standards | |||
| Area | Concentration (ppm) | ||
| 0 | 0 | ||
| 8.181 | 0.005 | ||
| 22.172 | 0.01 | ||
| 69.616 | 0.025 | ||
| 147.772 | 0.05 | ||
| Slope | Y-Intercept | ||
| 3025.9 | -4.9188 |
Beginning in late 2019, we began to harvest Inland Silverside tissues for culture and subsequent immortalization. Hearts, vertebrae, and liver explants were cultured for 48-72 hours and then enzymatically dissociated. Cells were plated in 24-well plates, and cardiomyocytes and putative osteoblastic cells successfully adhered. In early 2020, these cells were exposed to media containing lentiviral particles for immortalization with large T-antigen. These cells were maintained until February, when Candida yeast contamination resulted in loss of all cells. The COVID-19 pandemic prevented re-addressing of harvesting, dissociation, plating, and contamination until late April 2020 and delays continued until fall 2020. Yeast contamination persisted throughout the summer, irrespective of the presence of antifungal treatments in the media. Purchase of new media and extensive decontamination procedures were undertaken this fall, and harvesting and culture has resumed. Antifungal-resistant yeast contamination is still present in small amounts, but changes in culture protocols (e.g. buffer used, immortalization step moved before dispersal) has resulted in minimization of this contamination. Currently, cells are prepared again for immortalization within the next month.
Figure 2.
Figure 3. Image of Fibroblasts and Cardiomyocytes from M. beryllina following preliminary application of immortalization protocol.
Future Activities:
We are in the process of preparing a manuscript, led by Ph.D. student Sara Hutton, for a special issue of the journal Toxics on agrochemicals in aquatic ecosystems, co-edited by Brander and previous EPA co-I Dr. Alvine Mehinto. This year’s work includes additional validation of protocols for biocide extraction from water and embryos, immortalization of cell lines (cardiomyocytes, osteocytes, and hepatocytes) from M. beryllina tissues, as well as in vivo and in vitro exposures. Although delays related to Covid will likely continue to impact research progress until vaccines are widely available, as a team we have implemented successful work-arounds via the use of staggered schedules to reduce densities in the laboratory, and protective protocols (masks, additional sanitation procedures). We are optimistic about 2021.
Journal Articles:
No journal articles submitted with this report: View all 9 publications for this projectSupplemental Keywords:
Menidia beryllina, in vivo, in vitro, salinity, biocide, cell lineProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final
- 2024 Progress Report
- 2023 Progress Report
- 2022 Progress Report
- 2021 Progress Report
- Original Abstract
8 journal articles for this project