Grantee Research Project Results
Final Report: Reusable Biodegradable Solvents from Biodiesel
EPA Grant Number: SV839351Title: Reusable Biodegradable Solvents from Biodiesel
Investigators: Ott, Lisa
Institution: California State University - Chico
EPA Project Officer: Page, Angela
Phase: II
Project Period: March 1, 2018 through February 29, 2020 (Extended to May 31, 2022)
Project Amount: $73,338
RFA: P3 Awards: A National Student Design Competition for Sustainability Focusing on People, Prosperity and the Planet - Phase 2 (2017) Recipients Lists
Research Category: P3 Awards , Sustainable and Healthy Communities , P3 Challenge Area - Chemical Safety
Objective:
The goals of Phase II of this project were:
- Develop a process for rapid evaluation of cogenerated glycerol quality.
- Develop a more sustainable method for purifying glycerol from BC biodiesel synthesis.
- Reduce the amount of waste glycerol entering the waste stream.
- Develop DESs with the potential for scaled-up production by preparing and characterizing multiple series of DESs produced from a variety of HBAs.
- Screen and then optimize organic reaction in the DESs referenced above.
- Pilot the use of DESs developed in our research lab as solvents in the Organic Chemistry teaching labs at CSU Chico, then extend this use to the CSU campus in Pomona.
- Expand the economic analysis to consider the viability of and quantify the net benefit of using DESs in place of traditional solvents throughout the CSU system.
- Use two measures of risk—one using Bureau of Labor Statistics data and one using survey results-- to determine the nonmarket value of reducing students’ exposure to volatile organic compounds throughout the CSU system.
- Develop methods to determine nonmarket values associated with reduced solvent exposure
- Integrate ethical research and sustainability principles into educational experience
- Disseminate results through publications and presentations.
- Develop DESs with the potential for scaled-up production by preparing and characterizing multiple series of DESs produced from a variety of HBAs.
- Screen and then optimize organic reaction in the DESs referenced above.
- Pilot the use of DESs developed in our research lab as solvents in the Organic Chemistry teaching labs at CSU Chico, then extend this use to the CSU campus in Pomona.
- Expand the economic analysis to consider the viability of and quantify the net benefit of using DESs in place of traditional solvents throughout the CSU system.
- Use two measures of risk—one using Bureau of Labor Statistics data and one using survey results-- to determine the nonmarket value of reducing students’ exposure to volatile organic compounds throughout the CSU system.
- Develop methods to determine nonmarket values associated with reduced solvent exposure
- Integrate ethical research and sustainability principles into educational experience
- Disseminate results through publications and presentations.
The three main objectives for the proposed project were:
Protecting the environment: We will help protect the environment by designing a solution that: effectively uses materials and energy, reduces pollution and waste, eliminates toxic waste, and reuses chemicals created during the biodiesel manufacturing process. We will develop a process for transforming the glycerol that is less energy intensive and uses materials more efficiently than the current method for refining the waste glycerol.
Advancing economic competitiveness: DESs produced within the CSU system creates both market (decreased solvent purchase costs) and nonmarket (reduced exposure to traditional solvents) benefits. We will conduct an analysis of potential profits associated with producing DES from waste glycerol and review the extent that it is transferable to other entities; these include biodiesel producers and commercial/industrial consumers of solvents.
Improving human wellbeing: This research will help make biodiesel a more sustainable and accessible option for consumers. This project will also educate students and the public about renewable energy, green chemistry, and sustainability.
Summary/Accomplishments (Outputs/Outcomes):
Objective 1: Measure viscosity with the microscale viscometer.
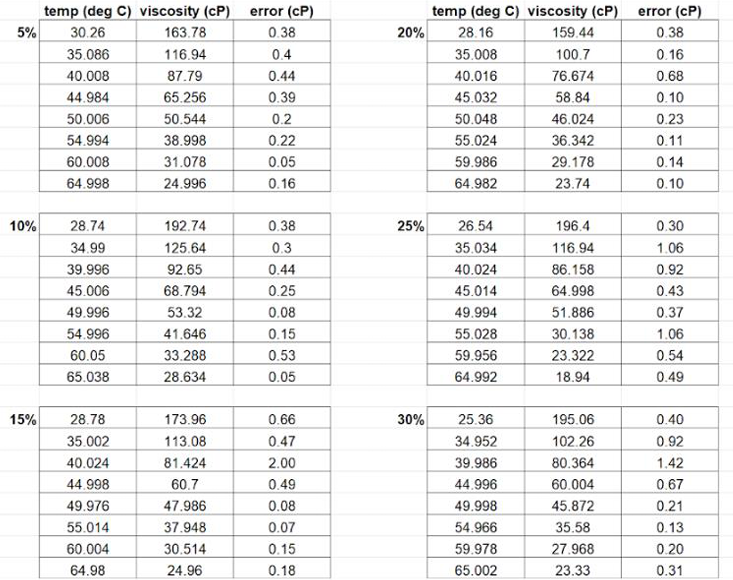
Getting the microscale viscometer to function properly took several years of effort by a number of students and two return trips to the manufacturer for reprogramming and repairs. However, since the measurement of viscosity is one of two essential indicators of the formation of a DES instead of a simple mixture, making these measurements was essential. Undergraduate chemistry major Radha Joshi was one of the two final students supported by this grant; she successfully completed the suite of measurements on DESs prepared from acidified base-catalyzed coproduct glycerol. Her data is shown below, in both tabular and graphical form.
Figure 1: Tabular format of data from undergraduate chemistry major Radha Joshi.
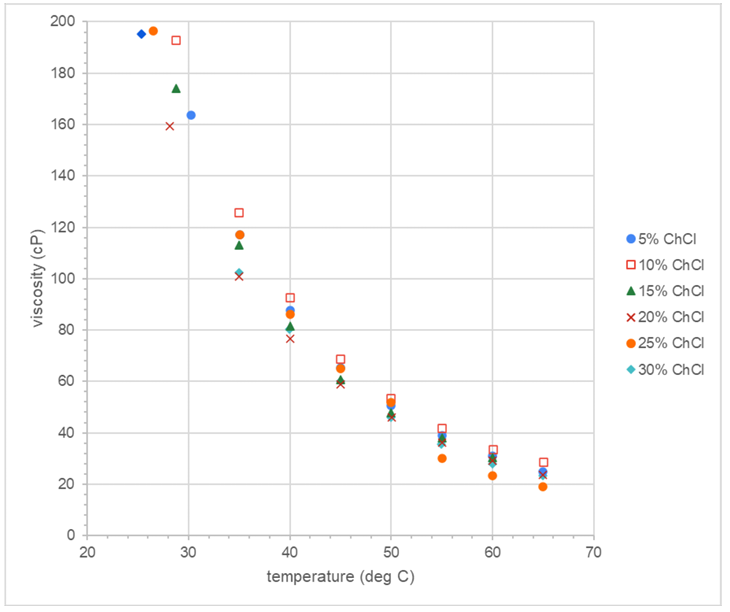
Figure 2: Graphical format of data from undergraduate chemistry major Radha Joshi.
As expected, the viscosity of each DES decreased with increasing temperature. Interestingly, at each individual temperature the trend was not straightforward with respect to the percentage of HBA. From the literature with synthetic (pure) glycerol, a trend of decreasing viscosity with increasing percentage of the HBA ChCl was observed. In our case, it seems that the 20% ChCl was the least viscous mixture at every temperature. That may indicate that 20% ChCl is the optimum percentage of the HBA for preparing DESs to be used in organic chemistry teaching laboratories.
The microscale viscometer was also used as a tool to rule out possible HBDs in new potential DES formation. For instance, the measurement of viscosity on a range of 5-30% betaine in acidified BC glycerol revealed a consistently increasing viscosity with increasing weight percent HBD. These measurements indicate the formation of a simple mixture, not a DES. Therefore, betaine was eliminated as a potential DES using co-product glycerol from acidified base-catalyzed biodiesel preparation.
Many more measurements with the microscale viscometer still need to be completed. For instance, sulfuric acid is another good candidate for salting out impurities and adjusting the pH of the BC glycerol. Since the viscometer is now functional, this work will continue and build into future funding opportunities.
Objective 2: Investigate the utility of our DESs as reaction solvent.
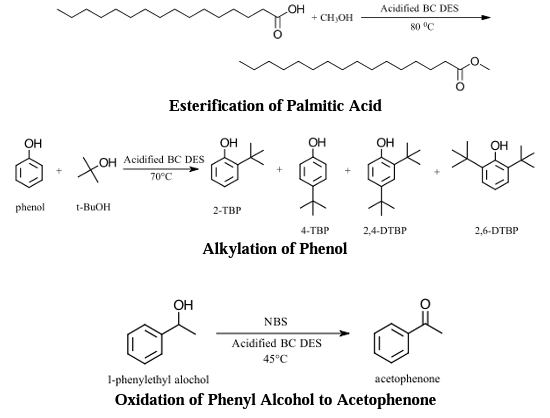
A variety of reactions were attempted in DESs to investigate the suitability of replacing traditional organic solvents in organic chemistry teaching laboratories. The reactions that were closely studied were the palmitic acid esterification which converted palmitic acid to methyl palmitate, the alkylation of phenol which converted the phenol into four alkylated products, and the oxidation of phenyl alcohol which converted the phenyl alcohol into a ketone. All three of these reactions went through a total of five runs and the DES was reused in each run. The reaction schemes for the three successful reactions are shown below:
Figure 3: Reaction schemes for the three successful reactions.
Each of these reactions are excellent candidates for substitution into the organic chemistry teaching laboratories. Product formation was carried out with 1H NMR and gas chromatography with mass spectrometric detection (GC-MS), two techniques that are essential in organic chemistry curricula. Consultation with the organic chemistry teaching faculty in future semesters will determine which of these reactions best fits into the curriculum the best (see below).
Objective 3: Start using DESs in the organic chemistry teaching labs.
Several factors conspired to make this objective unreachable during the course of the grant funding. We have developed three reactions which would be suitable for use in the organic teaching laboratories. However, the global pandemic completely shut down in-person laboratories from March 2020 through August 2021 at Chico State. At our collaborating institution, California Polytechnic University, Pomona (CPP), a return to in-person laboratories did not occur until February 2022. Therefore, integrating new laboratories into the curriculum CPP was not possible. At Chico State, in addition to the challenges posed by the pandemic, the chemistry department moved to a new building across campus. After the move the stockroom personnel asked the faculty to please not change laboratories as they attempted to move in, set up all of the teaching labs, settle their new stockrooms, and set up for in-person instruction with social distancing and physical barriers between students still in place. Therefore, we were not able to integrate the new DES reactions into the curriculum at Chico State either.
Objective 4: Characterize glycerol cogenerated with BC biodiesel and use as a HBD.
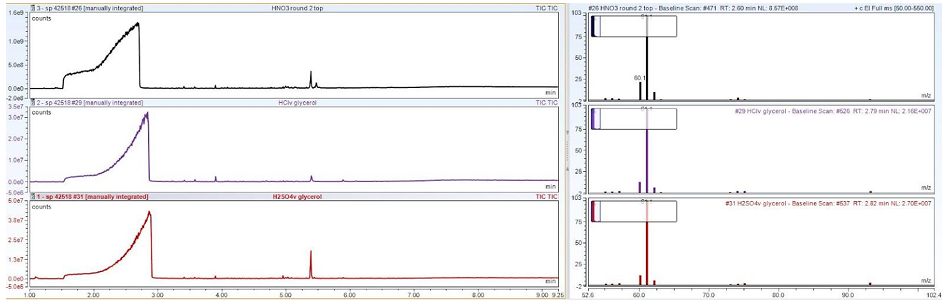
This was an objective which changed during the course of the project. Initially, our hypothesis was that the water content was an important metric for converting byproduct glycerol into DESs; we aimed to quantitate the water content using Karl Fischer titration. However, with the acidification process developed during the course of this work, determination of the water content was no longer an important parameter. Any salts present are precipitated with the addition of acid, and water was removed with the use of rotary evaporation. Then, characterization is simple by means of 1H NMR and GC-MS. Three GC-MS traces are shown below using three different acids used to purify the co-generated glycerol from BC biodiesel preparation. The large peak between 2-3 minutes is glycerol; the small peak around 5.5 minutes is trace biodiesel remaining in the co-product glycerol.
Figure 4: Three GCMS traces are shown using three different acids used to purify the co-generated glycerol from BC biodiesel preparation.
Objective 5: Train students in research and development of a product.
This grant supported eleven chemistry research students (10 undergraduate, one high school student) and one economics student. The chemistry students supported were Kelly Burns, Eric Fleischmann, Jordan Pittman (high school scholar), Annie Valceschini, Robin Bumbaugh, Shannon Price, Stephen Proctor, Christopher Lupton, Nirvana Almada, Radha Joshi, and Julia Penick. All of these students participated in independent research during the semester; seven of them also participated in our Chemistry Summer Research Institute, during which time they carried out full-time research. Several of these students earned positions in industry or graduate school based on the skills they developed during this Phase II supported work. Faith Fatchen completed Phase II economics work, building upon the analyses completed by Kylie Taylor and Amber Williams with our Phase I funding. Fatchen completed a non-market analysis of the environmental and health-related benefits of using DESs, working on the project full time in summer 2019 and part time for research credit in our department thereafter.
Objectives 6 & 7: Cost- benefits analysis of DES production to evaluate reuse of DES (6) and Evaluate the potential net benefits associated with scaling up production of DESs (7)
Using Phase I funding, economics students conducted a cost-benefit analysis of Phase I DES solvents, showing that they could be produced in a cost-efficient manner, even without consideration of non-market (environmental, health, and safety-related) benefits. In year 2 of Phase II work, economics student Faith Fatchen fielded a contingent ranking (choice experiment) survey to put monetary values on the non-market benefits associated with DES’ replacement of traditional solvents. The survey was given to freshman chemistry students over the Fall 2019 and start of Spring 2020 semesters. In year 3, Fatchen estimated econometric models that determine the dollar values associated with reducing potential risks of working with traditional solvents--i.e., the nonmarket and environmental values of producing DES. We find that DES have significant non-market benefits; results show students’ willingness to pay approximately $10 per semester to reduce environmental risks (associated with disposal) of traditional solvents. Students were willing to pay roughly $5 per semester to reduce risk associated with solvents’ flammability and potential for eye/skin damage. Thus, scaling up production of DES could lead to significant non-market benefits, given that roughly 100 students take Chemistry 270 (the class where DESs would be used) at Chico State each semester. A full description of the survey work and estimation techniques for estimating nonmarket benefits is included in Section 2 below. Unfortunately, Faith Fatchen was unable to incorporate new solvents developed during Phase II into a cost-benefits analysis, given the unfortunate delays for the chemistry students caused by the pandemic-related closures. However, all inputs used in the successful reactions tested by the chemistry students in Phase II have costs comparable (or equivalent) to the inputs used in Phase I; thus, we know that using DESs to replace traditional solvents is cost effective at all levels where this practice could be implemented (i.e., it would generate direct savings for both Chico State and for the university system as a whole). Incorporating the non-market benefits Fatchen estimated into the cost-benefit analysis only further increases the potential benefits (and thus benefit/cost ratio) of this project.
Journal Articles on this Report : 1 Displayed | Download in RIS Format
| Other project views: | All 8 publications | 1 publications in selected types | All 1 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Bumbaugh RE, Ott LS. Preparing and Testing Novel Deep Eutectic Solvents from Biodiesel Co-Product Glycerol for Use as Green Solvents in Organic Chemistry Teaching Laboratories. In Environmental Research Literacy:Classroom, Laboratory, and Beyond 2020; 113-130. |
SV839351 (2020) SV839351 (2021) SV839351 (Final) |
Exit |
Relevant Websites:
California State University Chico Center for Water and the Environment Exit
Progress and Final Reports:
Original AbstractP3 Phase I:
Reusable Biodegradable Solvents from Biodiesel | 2017 Progress Report | Final ReportThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- 2021 Progress Report
- 2020 Progress Report
- 2019 Progress Report
- 2018 Progress Report
- Original Abstract
- P3 Phase I | 2017 Progress Report | Final Report
1 journal articles for this project