Grantee Research Project Results
Final Report: Simultaneous Concentration and Real-time Detection of Multiple Classes of Microbial Pathogens from Drinking Water
EPA Grant Number: R833006Title: Simultaneous Concentration and Real-time Detection of Multiple Classes of Microbial Pathogens from Drinking Water
Investigators: Sobsey, Mark D. , Simmons, Otto D.
Institution: University of North Carolina at Chapel Hill
EPA Project Officer: Aja, Hayley
Project Period: October 18, 2006 through October 17, 2009
Project Amount: $599,999
RFA: Development and Evaluation of Innovative Approaches for the Quantitative Assessment of Pathogens in Drinking Water (2005) RFA Text | Recipients Lists
Research Category: Drinking Water , Water
Objective:
This project investigated the development and performance characteristics of methods to rapidly and efficiently recover and detect enteric viruses, pathogenic bacteria and parasites in drinking water samples. The specific objectives were: 1) to evaluate the newly developed NanoCeram electropositive cartridge filter for primary concentration of viruses from water; 2) to refine and validate new and improved rapid methods to concentrate and detect viruses as well as cellular pathogens (bacteria and protozoan parasites) from waters of different quality, 3) to evaluate rapid PEG precipitation and large volume nucleic acid extraction as post-filtration sample preparation techniques to further concentrate viruses and remove inhibitors for efficient, specific and sensitive real-time, molecular detection of viral nucleic acids from human adenoviruses, enteroviruses and noroviruses; 3) to optimize direct detection of viral RNA/DNA by real-time polymerase chain reaction (RT-PCR) for rapid and efficient detection of low numbers of target viruses; 4) to develop complete protocols of the methods and provide them to a select number of other water virology laboratories to conduct a collaborative (round-robin) test of the methods that characterizes their performance; and 5) to apply the new performance-validated method and the standard EPA method to selected field samples of raw water to document that the new methods give superior performance.
Summary/Accomplishments (Outputs/Outcomes):
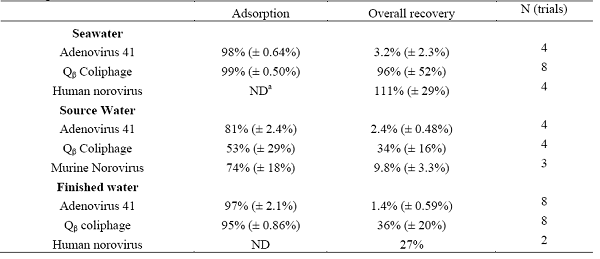
The NanoCeram cartridge filternow commercially available was evaluated as a primary concentration adsorbent filter for viruses in sea, source and finished water. Adsorption efficiencies of adenovirus and male-specific coliphages to the NanoCeram filter were high (>97%) from finished drinking water and from seawater. Viruses were eluted from the filters using a beef extract solution supplemented with 0.1 M of glycine at a pH of 9.5. For the efficient elution of norovirus and male-specific coliphages it was necessary to recirculate the elution buffer for 5 minutes with the aid of a peristaltic pump. The results on the adsorption and recoveries of viruses using the NanoCeram cartridge filter are presented in Table 1.
Table 1. Adsorption and recovery of viruses from different types of water using NanoCeram© cartridge filters.
The performance of continuous flow hollow fiber ultra filtration (HFUF) in the recovery of multiple classes of microbes was also intensively studied. Results demonstrated that the hollow fiber ultrafiltration procedure can effectively concentrate different types of microbes including viruses, bacteria and bacterial spores with recoveries > 50% (Table 2). The recoveries were similar in drinking source water and in drinking finished water.
Table 2. Microbial Recovery from Source and Dechlorinated Drinking Water Using HFUF with Lower Spikes of Microbial Indicators and Pathogens
- ND = No Data (eluting solution 2 with NaPP - toxic to cell culture)
Blank recoveries = <1%
Recovery percentage data for average and standard deviation
High versus low spike concentration Source Water Mann-Whitney p=0.6141 – No difference
High versus low spike concentration Drinking Water Mann-Whitney p=0.4494 – No difference
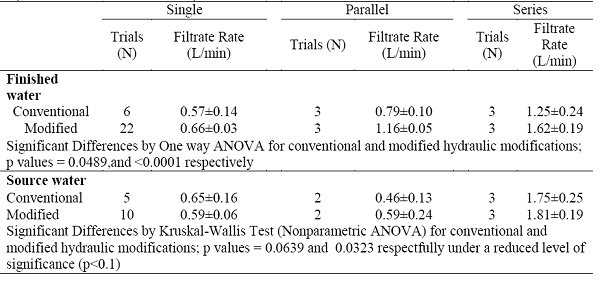
Modifications to the ultrafiltration flow patterns, such as the use of modified end-caps on the filter cartridge to increase inlet and outlet opening surface areas (cross-sections) and flux and the use of multiple filters in serial and parallel format, was investigated. No significant difference was found in the recovery of microbes using the different hydraulic modifications of ultrafiltration. However, the use of modified end-caps on the filter cartridge and the use of multiple HFUFs in serial format resulted in improved flow rates for the processing of drinking source water and drinking finished water (Table 3).
Table 3. Filtrate Rates of Fresenius F200A Ultrafilters in OWASA Treated Waters with Different Hydraulic Modifications to the System
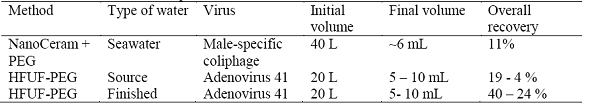
Polyethylene glycol (PEG) precipitation was optimized for the recovery of multiple classes of viruses and found to be a robust secondary concentration step for different eluants solutions and types of waters. A complete concentration method was demonstrated using adsorption-elution and PEG precipitation or using HFUF-PEG precipitation (Table 4).
Table 4. Performance of complete methods for the concentration of viruses from water
A Guanidinium thiocyanate (GuSCN) extraction method was demonstrated to be effective in the co-purification of nucleic acids from DNA and RNA viruses. In addition, different treatments were evaluated for the removal of inhibitory compounds from the concentrated samples facilitating sensitive viral detection. Although some treatments were very efficient in eliminating PCR inhibitory compounds from the concentrated samples, the selection of specific treatments to remove or overcome such inhibitors will depend on the nature of the sample and the type of virus being detected. No single method or set of methods for reducing sample inhibition of quantitative PCR was equally effective for both adenoviruses and noroviruses.
Conclusions:
The NanoCeram cartridge filters were found to be a highly efficient primary concentration method for male-specific coliphages and norovirus in large volumes of seawater. The advantages of the NanoCeram cartridge filter are its much lower price and its smaller size than the current standard EPA-approved electropositive cartridge filter. One can now filter the water in the field to concentrate viruses more conveniently and at lower cost than the currently approved filter. HFUF ultrafiltration was found to be an effective alternative primary concentration method and able to achieve universal concentration of not only viruses but also bacteria and bacterial spores from source and finished drinking waters. Hydraulic modification of the ultrafilters, such as using modified end-caps with greater inlet and out cross-sectional area on the filter cartridge and using multiple filters in series format increases the flow rate, resulting in shorter filtration times for large volumes of source and finished drinking waters. The advantage of HFUF is that microbial recovery effectiveness is not greatly affected by water conditions such as pH and turbidity. The main disadvantage is that the system is not as portable as the electropositive adsorbent filter for viruses. Therefore, the sample water typically needs to be shipped to a laboratory, resulting in extra time to process and analyze the sample before results are obtained and high shipping costs if large volumes of water are to be analyzed. PEG precipitation was found to be a robust and effective secondary concentration step for viruses. The combined use of NanoCeram filters with PEG precipitation can concentrate the water ~6,000 times and the use of HFUF with PEG precipitation can concentrate drinking water ~1000 times for sensitive detection of viruses from large volumes of water using real-time PCR. PCR inhibitors of virus detection in samples concentrated by both methods potentially can be reduced by treatments such as column chromatography and chloroform extraction, but no single method or set of conditions was found to be optimum for different viruses.
References:
Journal Articles on this Report : 1 Displayed | Download in RIS Format
| Other project views: | All 4 publications | 1 publications in selected types | All 1 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Gibbons CD, Rodriguez RA, Tallon L, Sobsey MD. Evaluation of positively charged alumina nanofibre cartridge filters for the primary concentration of noroviruses, adenoviruses and male-specific coliphages from seawater. Journal of Applied Microbiology 2010;109(2):635-641. |
R833006 (Final) |
Exit |
Supplemental Keywords:
Human viruses, Adenovirus, Norovirus, Concentration Large volumes of water, Real-time PCR, Universal Concentrator, RFA, Scientific Discipline, Water, Environmental Chemistry, Drinking Water, Environmental Engineering, microbial contamination, ultrafiltration, contaminant candidate list, pathogens, contaminant removal, drinking water contaminants, drinking water treatment, drinking water monitoring, CCL, detectionProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.