Grantee Research Project Results
2013 Progress Report: Neurodevelopment and Improving Children's Health following EtS exposure (NICHES)
EPA Grant Number: R835437Center: The Center for Study of Neurodevelopment and Improving Children's Health
Center Director: Murphy, Susan K.
Title: Neurodevelopment and Improving Children's Health following EtS exposure (NICHES)
Investigators: Murphy, Susan K. , Levin, Edward D , Kollins, Scott H , Engelhardt, Barbara , Fuemmeler, Bernard , Hall, Brandon , Hoyo, Cathrine , Seidler, Frederick , Satterwhite, Lisa , Slotkin, Theodore , Zhang, Weiwei
Current Investigators: Murphy, Susan K.
Institution: Duke University
EPA Project Officer: Hahn, Intaek
Project Period: June 1, 2013 through May 31, 2018 (Extended to May 31, 2019)
Project Period Covered by this Report: June 1, 2013 through May 31,2014
Project Amount: $3,907,780
RFA: Children's Environmental Health and Disease Prevention Research Centers (with NIEHS) (2012) RFA Text | Recipients Lists
Research Category: Children's Health , Human Health
Objective:
RD835437C001: Project 1 is examining the relationship between smoke exposure during early life and neurobehavioral outcomes in children followed from prior to birth through up to age 7 years, with a particular focus on attention deficit/hyperactivity disorder (ADHD). This project also is examining the relationship between smoke exposure, ADHD and DNA methylation. The objective of Project 1 is to evaluate the associations of both environmental tobacco smoke (ETS) exposure on cognitive and neurobehavioral outcomes across early development and examine the role of exposure-induced DNA methylation changes on these outcomes.
RD835437C002: Project 2 is determining how exposure during early development to tobacco smoke extract and to nicotine influences growth and neurobehavioral outcomes in rats and neural differentiation and neurotransmitter phenotypes in vitro. This project also is working to define the most sensitive developmental window(s) of vulnerability to tobacco smoke and nicotine exposure and will determine if dietary interventions can ameliorate the effects of these exposures.
RD835437C003: Project 3 is investigating how in utero tobacco smoke and nicotine exposure in rats influences DNA methylation in the brain and blood, and how the methylation profiles in the brain relate to gene expression. These findings are being applied to the study of cord blood from our human cohort to determine if methylation patterns can be used to stratify risk of ADHD prior to onset of symptoms. ADHD-associated genes also will be examined in the in vitro models of neurodifferentiation and neurotransmission to determine associations with these phenotypes.
Community Outreach and Translation Core is developing an educational primer about the effects of tobacco smoke on children, with particular emphasis on ADHD. The primer will be used as a tool to elicit interest in community participation in an Instagram contest that requires that the entry reflect what has been learned from the educational primer.
Specific aim 1: Develop a simple science primer that uses “lay language” to communicate the effects of ETS exposure (including nicotine) on the one’s genes (i.e., epigenetics). Similarly, communicate how these effects can be “handed down” to one’s offspring. Various stakeholders (e.g., doctors, pregnant patients, adolescent patients) will participate in the science primer development.
Specific aim 2: Disseminate the science primer in local community health centers to various stakeholders (e.g., doctors, pregnant patients, adolescent patients) along with a “call for contest applications” (see specific aim #4).
Specific aim 3: Develop a working document that translates the major findings of each of the three Center projects into “lay language” (add to the science primer in a section called “newest research”). Use the working document to develop the basis for a community-wide contest highlighting the basics about ETS and the newest research.
Specific aim 4: Hold a “contest.” Contestants (various stakeholders) use the science primer (from specific aim #2) along with the findings generated by the three Center projects (in specific aim #3) to construct their own YouTube videos and brochures that convey any of the NICHES findings. A committee of “judges” will choose a set of winning videos and brochures for a small field test and eventual dissemination.
Specific aim 5: Conduct a local field test in community and women’s health centers using the winning YouTube videos and brochures to determine the stakeholders’ attitudes and knowledge about the effects of ETS on themselves and their children.
Specific aim 6: Work with NIEHS to disseminate the YouTube videos and “science primers” within their database (and on the NIEHS website); post links to these materials online from the NICHES website and our science education website (www.rise.duke.edu), which has hundreds of thousands of hits.
Progress Summary:
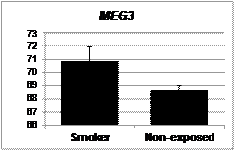
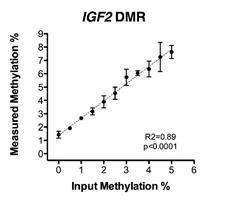
Future Activities:
- Finish neurobehavioral assessment for all cohorts of attention, learning, spatial working and reference memory, nonspatial memory, fear and anxiety for male and female rats exposed throughout early development to tobacco smoke extract (TSE) at a dose modeling environmental tobacco smoke exposure and the equivalent dose of nicotine (0.2 mg/kg/day) without the other constituents of TSE in comparison with vehicle control and a positive comparison group of a higher dose of nicotine (2 mg/kg/day), modeling the nicotine exposure from primary smoking. Determine the most sensitive neurobehavioral effects.
- Investigate effects of briefer exposures to determine specific time windows of development (preconception, early prenatal, late prenatal or neonatal) that are most important for causing neurobehavioral dysfunction.
- Begin assessments of pharmacotherapies to attenuate the neurobehavioral dysfunction caused by TSE and nicotine exposure during development.
- Perform brain regional dissections in an age range from birth through weaning, adolescence and adulthood of male and female rats exposed throughout development to TSE at a dose modeling environmental tobacco smoke exposure and the equivalent dose of nicotine (0.2 mg/kg/day) without the other constituents of TSE in comparison with vehicle control and a positive comparison group of a higher dose of nicotine (2 mg/kg/day), modeling the nicotine exposure from primary smoking.
- Perform brain region-specific neurochemical analyses characterizing monoaminergic and cholinergic neurotransmitter systems of in vivo tissues from perinatal TSE administration.
- Elucidate mechanisms underlying the TSE effects on neural cell replication, neurite outgrowth and differentiation in the PC12 model.
- Prepare TSE-exposed samples from PC12 studies for epigenetics evaluations.
- Characterize nicotine and TSE exposure phenotypes in human and rat neural stem cells (NSCs) by measures of EdU (S-phase), annexin V (apoptosis), propidium iodide (cell nonviability) and DAPI (cell number and mitotic index). Differentiation will be quantified by immunofluorescence and qPCR to identify neurons (MAP2, DCX), astrocytes (GFAP), oligodendrocytes (GALC), synaptic phenotypes (TH, ACh, AChR) and the undifferentiated state (NES); test the hypothesis that pretreatment with methyl donor micronutrients and antioxidants will rescue nicotine/TSE-associated exposure phenotypes. RNA and DNA will be isolated and archived for gene expression (Affymetrix human U133 Plus2.0 whole genome microarrays) and DNA methylation (Illumina Infinium HumanMethylation450BeadChip arrays) studies.
- Concordance between DNA methylation patterns in maternal/child whole blood (Project 1) and in human neural stem cells (hNSCs) exposed to nicotine/TSE will be determined in select loci and genome/epigenome-wide studies. We will assay individual loci associated with prenatal exposure to nicotine and ADHD (Project 3) and loci associated with the developmental dopaminergic specification pathway. including PITX3 (paired like homeobox domain 3), NR4A2 (Nurr1), SLC6A2 (norepinephrine transporter), SLC6A3 (dopamine transporter 1), DRD1-5 (dopamine receptors 1-5) and EN1 (engrailed homeobox 1).
- Identify targets of methylation in exposed humans and rats. We will progress with pyrosequencing and data analysis, adding additional genes for analysis, and with Sequenom screening to identify target regions in both humans and in rat tissues, as well as in cultured cells.
- Once tissues from exposed/unexposed and behaviorally characterized rats are available from Project 2, we will generate RNA and DNA; pooled RNA will be used for whole transcriptome profiling to determine how gene expression in frontal cortex is altered by tobacco smoke and nicotine exposures, and the pooled DNA will be used for whole genome bisulfite sequencing to determine how the methylome is altered, to be carried out using funds from years 2 and 3 (due to expense).
- The RNA from individual rat brain, liver and kidney tissues, as well as cultured cells, will be used to validate the expression levels detected by high-throughput transcriptome sequencing.
- Cotinine assays will be run from maternal blood for the individuals undergoing analysis in Project 3 to enable analysis of the methylation data with respect to a continuous marker of exposure.
- A human subjects protocol will be prepared for the North Carolina State Department of Health and Human Services Clinical Chemistry Laboratory to allow for analysis of lead levels in year 5.
- We will continue to bank and track biological specimens as they are collected from Projects 1 and 2.
Journal Articles: 34 Displayed | Download in RIS Format
| Other center views: | All 118 publications | 40 publications in selected types | All 34 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Breton CV, Marsit CJ, Faustman E, Nadeau K, Goodrich JM, Dolinoy DC, Herbstman J, Holland N, LaSalle JM, Schmidt R, Yousefi P, Perera F, Joubert BR, Wiemels J, Taylor M, Yang IV, Chen R, Hew KM, Hussey Freeland DM, Miller R, Murphy SK. Small-magnitude effect sizes in epigenetic end points are important in children’s environmental health studies: the Children’s Environmental Health and Disease Prevention Research Center’s Epigenetics Working Group. Environmental Health Perspectives 2017;125(4):511-526. |
R835437 (2017) |
|
|
|
Small Magnitude Effect Sizes in Epigenetic Endpoints are Important in Children's Environmental Health Studies:The Children's Environmental Health and Disease Prevention Research Center's Epigenetics Working Group. Environmental Health Perspectives 2017; 125(4):511-526. |
R835437 (2016) |
|
|
|
Cauley M, Hall BJ, Abreu-Villaca Y, Junaid S, White H, Kiany A, Slotkin TA, Levin ED. Critical developmental periods for effects of low-level tobacco smoke exposure on behavioral performance. Neurotoxicology 2018;68:81-87. |
R835437 (2017) |
Exit Exit Exit |
|
|
Fleisch AF, Kloog I, Luttmann-Gibson H, Gold DR, Oken E, and Schwartz JD. Air Pollution Exposure and Gestational Diabetes Mellitus Among Pregnant Women in Massachusetts:a Cohort Study. Environmental Health 2016; 15:40-48. |
R835437 (2016) R834798 (Final) R834798C005 (Final) |
Exit |
|
|
Fuemmeler BF, Wang L, Iversen ES, Maguire R, Murphy SK, Hoyo C. Association between prepregnancy body mass index and gestational weight gain with size, tempo, and velocity of infant growth: analysis of the Newborn Epigenetic Study cohort. Childhood Obesity 2016;12(3):210-218. |
R835437 (2015) |
Exit Exit Exit |
|
|
Fuemmeler BF, Lee CT, Soubry A, Iversen ES, Huang Z, Murtha AP, Schildkraut JP, Jirtle RL, Murphy SK, Hoyo C. DNA methylation of regulatory regions of imprinted genes at birth and its relation to infant temperament. Genetics and Epigenetics 2016;8:59-67. |
R835437 (2017) |
Exit Exit |
|
|
Fuemmeler B, Glasgow T, Schechhter J, Maguire R, Sheng Y, Bidopia T, Barsell D, Ksinan A, Zhang J, Lin Y, Hoyo C, Murphy S, Qin J, Wang X, Kollins S. Prenatal and Childhood Smoke Exposure Associations with Cognition, Language, and Attention-Deficit/Hyperactivity Disorder. JOURNAL OF PEDIATRICS 2023;256:77 |
R835437 (Final) |
Exit |
|
|
Fuemmeler B, Dahman B, Glasgow T, Barsell D, Oliver J, Zhang J, Hoyo C, Murphy S, McClernon F, Wheeler D. Tobacco Exposures are Associated With Healthcare Utilization and Healthcare Costs in Pregnant Persons and Their Newborn Babies. NICOTINE & TOBACCO RESEARCH 2024; |
R835437 (Final) |
Exit |
|
|
Gao L, Liu X, Millstein J, Siegmund KD, Dubeau L, Maguire RL, Zhang JJ, Fuemmeler BF, Kollins SH, Hoyo C, Murphy SK, Breton CV. Self-reported prenatal tobacco smoke exposure, AXL gene-body methylation, and childhood asthma phenotypes. Clinical Epigenetics 2018;10(1):98 (11 pp.). |
R835437 (2017) |
Exit Exit Exit |
|
|
Hall BJ, Cauley M, Burke D, Kiany A, Slotkin TA, Levin ED. Cognitive and behavioral impairments evoked by low-level exposure to tobacco smoke components: comparison with nicotine alone. Toxicological Sciences 2016;151(2):236-244. |
R835437 (2015) |
Exit |
|
|
Hall BJ, Abreu-Villaca Y, Cauley M, Junaid S, White H, Kiany A, Levin ED. The ventral hippocampal muscarinic cholinergic system plays a key role in sexual dimorphisms of spatial working memory in rats. Neuropharmacology 2017;117:106-113. |
R835437 (2017) |
Exit Exit |
|
|
Hoffman K, Butt C, Webster T, Preston E, Hammel S, Makey C, Lorenzo A, Cooper E, Carignan C, Meeker S, Price T, Hoyo C, Mendelsohn E, Congleton J, Daniels J, Stapleton H. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. ENVIRONMENTAL SCIENCE & TECHNOLOGY LETTERS 2017;4(3):112-118. |
R835437 (Final) |
Exit Exit |
|
|
King KE, Kane JB, Scarbrough P, Hoyo C, Murphy SK. Neighborhood and family environment of expectant mothers may influence prenatal programming of adult cancer risk: discussion and an illustrative DNA methylation example. Biodemography and Social Biology 2016;62(1):87-104. |
R835437 (2015) |
Exit |
|
|
Lee W-C, Shen L, Catalano PJ, Mickley LJ, and Koutrakis P. Effects of Future Temperature Change on PM2.5 Infiltration in the Greater Boston Area. Atmospheric Environment 2017;150:98-105. |
R835437 (2016) R834798 (Final) R835755 (2016) |
Exit Exit Exit |
|
|
Levin ED. Learning about cognition risk with the radial-arm maze in the developmental neurotoxicology battery. Neurotoxicology and Teratology 2015;52(Pt A):88-92. |
R835437 (2014) |
Exit Exit Exit |
|
|
Murphy SK, Erginer E, Huang Z, Visco Z, Hoyo C. Genotype-epigenotype interaction at the IGF2 DMR. Genes 2015;6(3):777-789. |
R835437 (2014) |
Exit Exit Exit |
|
|
Nye MD, Fry RC, Hoyo C, Murphy SK. Investigating epigenetic effects of prenatal exposure to toxic metals in newborns: challenges and benefits. Medical Epigenetics 2014;2(1):53-59. |
R835437 (2013) R835437 (2014) |
Exit Exit Exit |
|
|
Nye MD, Hoyo C, Murphy SK. In vitro lead exposure changes DNA methylation and expression of IGF2 and PEG1/MEST. Toxicology In Vitro 2015;29(3):544-550. |
R835437 (2014) |
Exit Exit Exit |
|
|
Nye MD, King KE, Darrah TH, Maguire R, Jima DD, Huang Z, Mendez MA, Fry RC, Jirtle RL, Murphy SK, Hoyo C. Maternal blood lead concentrations, DNA methylation of MEG3 DMR regulating the DLK1/MEG3 imprinted domain and early growth in a multiethnic cohort. Environmental Epigenetics 2016;2(1):1-8. |
R835437 (2015) |
Exit Exit |
|
|
Schechter JC, Kollins SH. Prenatal smoke exposure and ADHD: advancing the field. Pediatrics 2017;139(2):e20163481 (2 pp.). |
R835437 (2017) |
Exit Exit Exit |
|
|
Schechter JC, Fuemmeler, BF, Hoyo C, Murphy SK, Zhang JJ, Kollins SH. Impact of smoking ban on passive smoke exposure in pregnant non-smokers: using cotinine as a biomarker of exposure. International Journal of Environmental Research and Public Health 2018;15(1):E83 (16 pp.). |
R835437 (2017) |
Exit Exit Exit |
|
|
Slotkin TA, Card J, Seidler FJ. Adverse benzo[a]pyrene effects on neurodifferentiation are altered by other neurotoxicant coexposures: interactions with dexamethasone, chlorpyrifos, or nicotine in PC12 cells. Environmental Health Perspectives 2013;121(7):825-831. |
R835437 (2013) R835437 (2014) |
|
|
|
Slotkin TA, Card J, Seidler FJ. Nicotine administration in adolescence reprograms the subsequent response to nicotine treatment and withdrawal in adulthood: sex-selective effects on cerebrocortical serotonergic function. Brain Research Bulletin 2014;102:1-8. |
R835437 (2014) |
Exit Exit Exit |
|
|
Slotkin TA, Card J, Stadler A, Levin ED, Seidler FJ. Effects of tobacco smoke on PC12 cell neurodifferentiation are distinct from those of nicotine or benzo[a]pyrene. Neurotoxicology and Teratology 2014;43:19-24. |
R835437 (2013) R835437 (2014) |
Exit Exit Exit |
|
|
Slotkin TA, Skavicus S, Card J, Levin ED, Seidler FJ. Amelioration strategies fail to prevent tobacco smoke effects on neurodifferentiation: nicotinic receptor blockade, antioxidants, methyl donors. Toxicology 2015;333:63-75. |
R835437 (2014) |
Exit Exit Exit |
|
|
Slotkin TA, Skavicus S, Card J, Stadler A, Levin ED, Seidler FJ. Developmental neurotoxicity of tobacco smoke directed toward cholinergic and serotonergic systems: more than just nicotine. Toxicological Sciences 2015;147(1):178-189. |
R835437 (2014) |
Exit |
|
|
Slotkin TA, Stadler A, Skavicus S, Seidler FJ. Adolescents and adults differ in the immediate and long-term impact of nicotine administration and withdrawal on cardiac norepinephrine. Brain Research Bulletin 2016;122:71-75. |
R835437 (2015) |
Exit Exit Exit |
|
|
Slotkin TA, Skavicus S, Card J, Levin ED, Seidler FJ. Diverse neurotoxicants target the differentiation of embryonic neural stem cells into neuronal and glial phenotypes. Toxicology 2016;372:42-51. |
R835437 (2016) R835437 (2017) |
Exit Exit Exit |
|
|
Slotkin TA, Stadler A, Skavicus S, Card J, Ruff J, Levin ED, Seidler FJ. Is there a critical period for the developmental neurotoxicity of low-level tobacco smoke exposure? Toxicological Sciences 2017;155(1):75-84. |
R835437 (2017) |
Exit Exit Exit |
|
|
Slotkin, T.A., Stadler, A., Skavicus, S., Card, J., Ruff, J., Levin, E.D., Seidler, F.J. 2016. Is there a critical period for the developmental neurotoxicity of low-level tobacco smoke exposure? Toxicological Sciences. DOW:10.1093. |
R835437 (2016) |
not available |
|
|
Smeester L, Yosim AE, Nye MD, Hoyo C, Murphy SK, Fry RC. Imprinted genes and the environment: links to the toxic metals arsenic, cadmium, lead and mercury. Genes 2014;5(2):477-496. |
R835437 (2014) |
Exit Exit Exit |
|
|
Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. BioEssays 2014;36(4):359-371. |
R835437 (2013) R835437 (2014) |
Exit Exit Exit |
|
|
Tindula G, Murphy SK, Grenier C, Huang Z, Huen K, Escudero-Fung M, Bradman A, Eskenazi B, Hoyo C, Holland N. DNA methylation of imprinted genes in Mexican-American newborn children with prenatal phthalate exposure. Epigenomics 2018;10(7):1011-1026. |
R835437 (2017) |
Exit Exit |
|
|
Vidal AC, Benjamin Neelon SE, Liu Y, Tuli AM, Fuemmeler BF, Hoyo C, Murtha AP, Huang Z, Schildkraut J, Overcash F, Kurtzberg J, Jirtle RL, Iversen ES, Murphy SK. Maternal stress, preterm birth, and DNA methylation at imprint regulatory sequences in humans. Genetics and Epigenetics 2014;6:37-44. |
R835437 (2014) |
Exit Exit Exit |
Supplemental Keywords:
secondhand smoke, nicotine, tobacco smoke extract, attention deficit hyperactivity disorder, neurobehavior, cognitive function, neurotransmission, human neural stem cells, pyrosequencing, DNA methylation, epigenetics, InstagramRelevant Websites:
http://sites.duke.edu/helpbabiesavoidsmoke Exit
Progress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2017 Progress Report
- 2016 Progress Report
- 2015 Progress Report
- 2014 Progress Report
- Original Abstract
34 journal articles for this center
