Cycling and Transformation
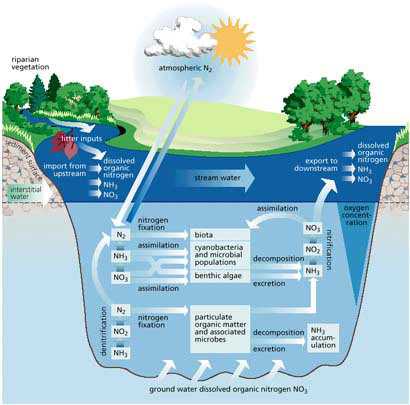
Cycling and transformation are another broad class of natural functions in watersheds. Various elements and materials (including water) are in constant cycle through watersheds, and their interactions drive countless other watershed functions. The figure at left, for example, illustrates interactions of the carbon and nitrogen cycles with stream biota and the resulting influence on dissolved oxygen. Elements like carbon, nitrogen, and phosphorus comprise the watershed's most important biogeochemical cycles. Cycling involves an element of interest's transport and storage, change in form, chemical transformation and adsorption.
Nutrient Spiraling. The flow of energy and nutrients in ecosystems are cyclic, but open-ended. True systems, in both an environmental and energetic context, are either "open" (meaning that there is some external input and/or output to the cyclic loop) or "closed" (meaning that the system is self-contained). In watersheds, streams and rivers represent an open-system situation where energy and matter cycles, but due to the unidirectional flow, the matter does not return to the spot from whence it came. Also, nutrients "spiral" back and forth among the water column, the bodies of terrestrial and aquatic organisms, and the soil in the stream corridor en route downstream. Hence, the concept of nutrient "spiraling" implies both movement downstream and multiple exchanges between terrestrial and aquatic environment, as well as between biotic and abiotic components of the watershed.
The Cycling of Carbon and Energy. In food webs, carbon and the subsequent synthesized energy is cycled through trophic (food web) levels. Energy transfer is considered inefficient, with less than 1% of the usable solar radiation reaching a green plant being typically synthesized by consumers, and a mere 10% of energy being typically converted from trophic level to trophic level by consumers.
Nitrogen (N). N2 (gaseous state) is not usable by plants and most algae. N-fixing bacteria or blue-green algae transform it into nitrite (NO2) or ammonia (NH4). N-fixation, precipitation, surface water runoff, and groundwater are all sources of nitrogen. Under aerobic conditions, NH4+ is oxidized to NO3- (nitrate) in the nitrification process. Losses of N occur with stream outflow, denitrification of nitrate (NO3) to N2 by bacteria, and deposition in sediments. Unlike P, inorganic N ions are highly soluble in water and readily leach out of soils into streams. NH4+ (ammonium) is the primary end-product of decomposition.
Phosphorus (P). Phosphorus in unpolluted watersheds is imported through dust in precipitation, or via the weathering of rock. Phosphorus is normally present in watersheds in extremely small amounts; usually existing dissolved as inorganic orthophosphate, suspended as organic colloids, adsorbed onto particulate organic and inorganic sediment, or contained in organic water. Soluble reactive phosphorus (consisting of ionic orthophosphates) is the only significant form available to plants and algae and constitutes less than 5% of the total phosphorus in most natural waters. Phosphorus tends to exist in waters of a pH of 6-7. At a low pH (<6), P tends to combine readily with manganese, aluminum, and iron. At a higher pH (>7), P becomes associated with calcium as apatite and phosphate minerals. It is normally retained in aquatic systems by algae, bacteria and fungi.
Nitrogen and Phosphorus limitation. Most watershed systems (both the aquatic and terrestrial realms) are either N or P limited, in that these are the required elements which are at the lowest availability. As a general rule, the N:P ratio should be 15:1. A lower ratio would indicate that N is limiting, a higher ratio places P in that role. Commonly P is the limiting factor. Often, the slightest increase in P can trigger growth, as in algal blooms in an aquatic setting. In N and P limited systems, an input of either element above and beyond normal, "natural" levels may lead to eutrophication.
The stream corridor is often a mediator of upland-terrestrial nutrient exchanges. As N and P move down through subsurface flow, riparian root systems often filter and utilize N and P, leaving less to reach the stream. This has a positive influence on those already nutrient-overloaded bodies of water, but would not necessarily be a positive influence on organisms struggling to find food in very clean, nutrient-limited headwaters streams. Microbes also denitrify significant amounts of N to the atmosphere. Still, N-fixers, like alder, may serve as sources of N for the stream channel, and groundwater pathways between the stream and the streamside forest may provide significant quantities of nitrogen.
Decomposition. Decomposition involves the reduction of energy-rich organic matter (detritus), mostly by microorganisms (fungi, bacteria, and protozoa) to CO2, H2O and inorganic nutrients. Through this process they both release nutrients available for other organisms and transform organic material into energy usable by other organisms. In lakes, much of the decomposition occurs in the waters prior to sedimentation. In the headwater reaches of streams, external sources of carbon from upland forests are a particularly important source of organic material for organisms and decomposition of microscopic particles occurs very rapidly. The bacteria and fungi modify the organic material through decomposition and make it an important food source for invertebrate and vertebrate detritivores, thereby reinserting these nutrients and materials into the watershed's aquatic and terrestrial food webs.
Decomposition is influenced by moisture, temperature, exposure, type of microbial substrate, vegetation, etc. Specifically, temperature and moisture affect the metabolic activity on the decomposing substrate. Nutritional value (as well as palatability) of the decomposing structure will also affect the time involved in complete breakdown and mineralization. Decomposition involves the following processes:
- The leaching of soluble compounds from dead organic matter
- Fragmentation
- Bacterial and fungal breakdown
- Consumption of bacterial and fungal organisms by animals
- Excretion of organic and inorganic compounds by animals
- Clustering of colloidal organic matter into larger particles
![[logo] US EPA](https://www.epa.gov/epafiles/images/logo_epaseal.gif)