Grantee Research Project Results
Final Report: BioWinol Technologies: A hybrid green process for biofuel production – Phase 2
EPA Grant Number: SU835171Title: BioWinol Technologies: A hybrid green process for biofuel production – Phase 2
Investigators: Wilkins, Mark , Dharman, Karthikeyan Ramachandriya , Liu, Kan , Atiyeh, Hasan , Huhnke, Raymond , Zhu, Xiaoguang , Kundiyana, Dimple , Terrill, Jennine
Institution: Oklahoma State University
EPA Project Officer: Page, Angela
Phase: II
Project Period: August 15, 2011 through August 14, 2013
Project Amount: $75,000
RFA: P3 Awards: A National Student Design Competition for Sustainability Focusing on People, Prosperity and the Planet - Phase 2 (2011) Recipients Lists
Research Category: Pollution Prevention/Sustainable Development , P3 Challenge Area - Air Quality , P3 Awards , Sustainable and Healthy Communities
Objective:
Every year, the United States energy generation sector releases billions of metric tons of carbon dioxide into the atmosphere [1]. This is contributing to significant amounts of global warming and change in climate patterns [1, 2]. One likely solution to abate the issue of carbon dioxide release to the atmosphere is to capture and utilize the emissions from power plants and other large point sources for production of fuels and chemicals. Among the numerous technologies under development for carbon capture [3], the most sustainable process will be to produce fuels and chemicals using microorganisms. Algal systems are a promising source for CO2 sequestration [4, 5], but their sunlight requirements and enormous water usage are the major constraints with the development of an economic process [6]. Alternatively, autotrophic microorganisms would provide an opportunity to produce fuels such as ethanol and butanol and industrial chemicals such as acetic acid and butyric acid without large land requirements.
The BioWinol Technologies project proposed a unique hybrid technology that uses several renewable resources, specifically biomass, wind and solar, to produce hydrogen (H2). This process can also capture carbon-dioxide (CO2) from other industries to produce biofuels. In Phase I, appropriate microorganisms were identified and media composition was developed for the process using small-scale lab experiments. In Phase II, a hollow fiber membrane (HFM) reactor was developed and tested using an organism identified in Phase I. Various pH control schemes were tested. Also, four PhD students developed various aspects of the process.
The major outcome of this project was a base process for production of biofuels and green commodity chemicals from CO2 and H2 obtained from integration of different renewable technologies. Information on process parameters and yields will guide future research and process design as well as economic analysis of this integrated process. The immediate benefit of this technology is applicable to power plants, cement plants, breweries and chemical industries for CO2 capture and conversion into both biofuels and green chemicals.
Our objectives were to 1) design and construct a lab-scale HFM reactor for conversion of CO2 and H2 to ethanol and 2) optimize reactor pH, gas flow rate and liquid flow rate to maximize ethanol production rate and concentration.
Summary/Accomplishments (Outputs/Outcomes):
The bacterium Clostridium carboxidivorans was selected based on Phase I results for further testing and scale-up from small serum bottle fermentations to larger lab-scale fermenters with temperature and pH control. Additionally, we decided to construct a lab-scale hollow fiber membrane (HFM) reactor in order to enhance the mass transfer of CO2 and H2 to the bacteria cells. Enhanced mass transfer will deliver more substrate to the cells and allow the productivity of the cells to increase. Mass transfer of gases to cells is limited in stirred tank reactors due to the low solubility of most gases in water and the resistance to transfer by the interface between gas bubbles and media. In a HFM system, gas diffuses through a membrane into the media, which avoids the resistance due to bubble media interface. A recent study comparing mass transfer coefficients for various fermenter types showed that hydrophobic HFMs have much mass transfer coefficients for gases that are over 4 times greater than hydrophilic membranes [7]. This was due to water not being able to enter the lumen of the membranes, which allowed gaseous diffusion to be the main mechanism of mass transfer as opposed to liquid diffusion. Also, the hydrophobic HFM reactors achieved mass transfer coefficients that were from 2 to 10 times greater than the maximum mass transfer coefficient observed in a stirred tank reactor [7].
Due to the high mass transfer coefficients observed for HFM fermenters, the BioWinol team decided to use this fermenter type to scale up the CO2/H2 fermentation. Several vendors were contacted to supply membranes, and two suppliers were identified, MedArray and Membrana. MedArray produces hydrophobic polydimethylsiloxane (PDMS) non-porous membranes and Membrana produces hydrophobic polypropylene porous membranes. Membranes were purchased from both companies and tested for durability, ease of use, and fermentation performance (cell growth and ethanol production). The PDMS membrane was smaller and more expensive than the PP membranes. Also, the PDMS membranes developed holes in them under pressure and good cell growth was not obtained. Additionally, a biofilm was observed to form on the PP membrane, which enables direct transfer of gas to the cells in the biofilm without diffusion into the media. For these reasons, the Membrana PP membrane was chosen for further study.
A HFM fermenter system was setup as shown in Figure 1. Gas containing 75% H2 and 25% CO2 entered the system through two HFMs at a rate of 20scc/min. Medium (2.5L) was placed in a 3L stirred vessel equipped with temperature and pH control. The medium was pumped through the HFMs at 300 mL/min in a direction counter to the gas flow. Medium contained (per L): 30 mL mineral stock solution, 10 mL trace metal solution, 10 mL vitamin solution, 30 g corn steep liquor (CSL), 10 mL of 4% cysteine sulfide solution, and 0.1 mL of 1% resazurin indicator. A 70g/L NaHSO3 solution was used to control pH. Two Aalborg gas flow meters were used to monitor inlet and outlet gas flow. The pressure in the fermentation system was 110 kPa, which was slightly above atmospheric pressure to prevent gas leaks.
Three experiments have been completed that have investigated the effect of pH on cell growth, product formation. A pH of 6.0 has been shown to promote cell growth in our previous work with C. carboxidivorans, while pH values from 4.5 to 5 have been shown to promote alcohol production. The first experiment (E1) controlled pH at 6.0 for 72 h, after which the pH was allowed to decline to pH 5.5. The second experiment (E2) controlled pH at 6.0 for the entire experiment. The third experiment (E3) allowed pH to decline from the initial pH of 5.8 to 5.5, and then the pH was controlled at 5.5. Cell growth, ethanol, acetic acid, n-butanol, n-butyric acid, n-hexanol and n-hexanoic acid were measured over time.
- Continuous stirred tank reactor (CSTR)
- Constant flow pump
- HFM vessel
- HFMs
- Gas (CO2/H2) inlet
- Gas outlet
- Liquid inlet conduit
- Liquid outlet conduit
Figure 1. BioWinol reactor setup.
Figure 2. Cell growth under various pH control schemes (see text for scheme descriptions).
Figure 3. Ethanol production under various pH control schemes (see text for scheme descriptions).
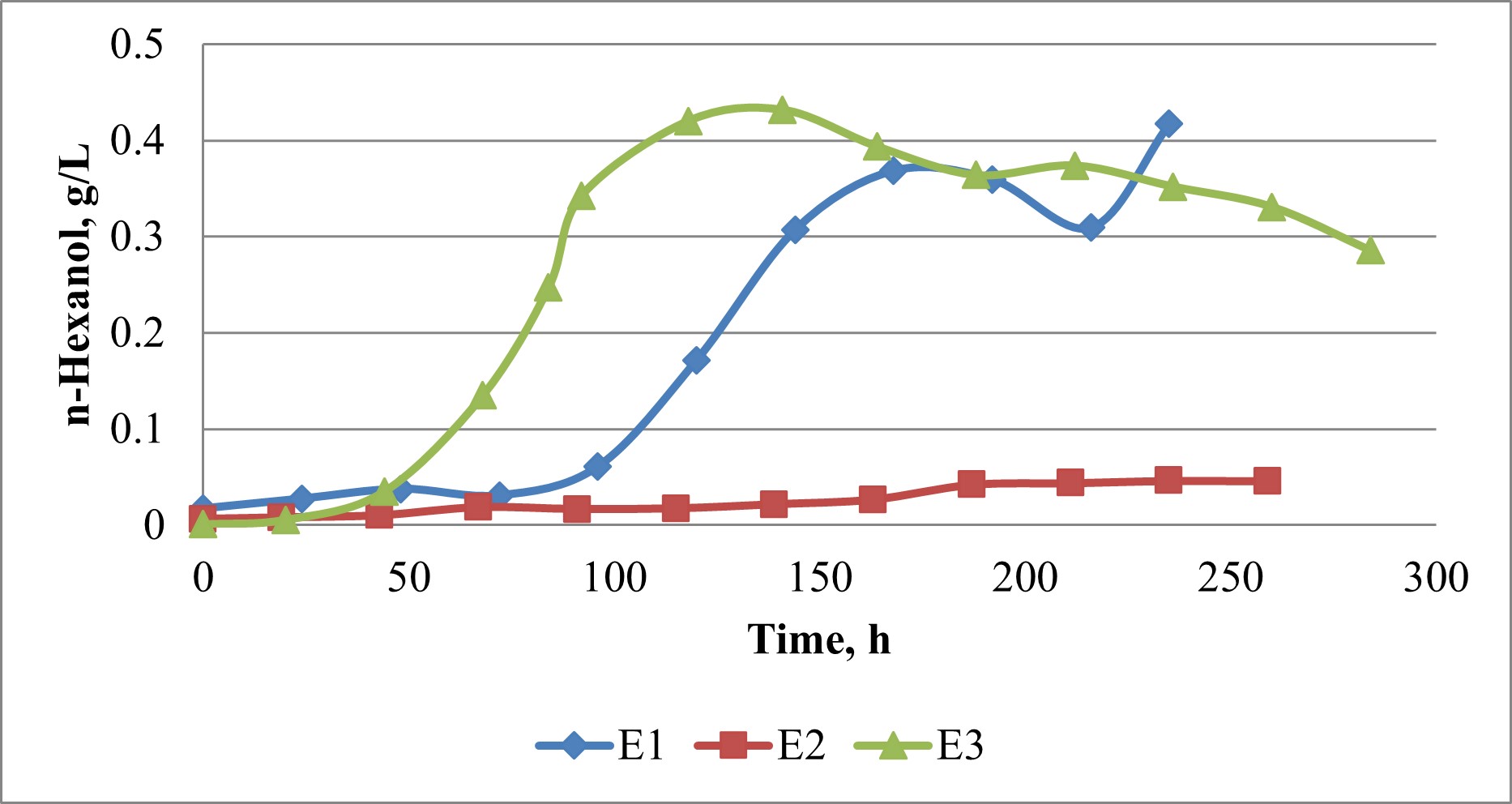
Figure 4. n-Hexanol production under various pH control schemes (see text for scheme descriptions).
The pH value did not have an effect on the initial growth rate of C. carboxidivorans (Figure 2). Cell growth in E3 was actually sustained longer than E1 and E2. This is contrary to the expectation that pH control at pH 6 would result in increased growth. Instead, it appeared cell growth may have been more dependent on the amount on nutrients available in the medium. The pH did have an effect on alcohol production. In both E1 and E2, ethanol production was observed in the first 48 to 50 h, and then ethanol was consumed by the cells or stripped from the medium by the gas flow (Figure 3). In E1 ethanol production resumed at 96 h once pH decreased to 5.5, while in E2, which had pH controlled at pH6, ethanol production resumed after 163 h, but a much lower rate than that observed with E1. In E3 ethanol was produced from 20 to 141 h. The pH in E3 had declined to 5.56 at 20 h, so it appears the reduction in pH to ~5.5 coincided with the start of ethanol production in both E1 and E2. n-Butanol and n-hexanol were also produced in all three fermentations. The trend for n-butanol production was similar to that of ethanol production for all three pH control schemes with butanol production being greatest for
E1 (0.83 g/L), followed by E3 (0.50 g/L) with little butanol being produced in E2 (data not shown). n-Hexanol production was produced at the same time as ethanol (Figure 4). Concentration of n-hexanol reached 0.43 g/L in both E1 and E3, which are greater than any n-hexanol concentrations reported in literature. These concentrations are quite remarkable given the fact that CO2 and H2 were the substrates. n-Hexanol is a potential fuel compound and it can also be converted to jet fuel through catalysis.
While we were able to achieve Objective 1, Objective 2 was not completed. The student that was responsible for carrying out Objective 2 was dismissed from Oklahoma State University (reasons for dismissal cannot be disclosed due to federal law). Additionally, the commissioning of the HFM system took several months longer than anticipated, which consumed time and money available from the grant. In the future, we hope to secure additional funding to optimize the HFM reactor design on the BioWinol and other gas fermentation designs.
Conclusions:
A hollow fiber membrane fermenter can be used to effectively transfer gas to cells. CO2 and H2 can be converted to ethanol, n-butanol and n-hexanol, all of which can be used as biofuels. The pH in the fermenter greatly affects the products formed with alcohol being produced at pH 5.5 in greater concentrations that at pH 6.0. We can successfully convert CO2 and H2 to valuable products, which would provide a means for energy storage from intermittent renewable energy generators such as wind turbines and solar energy installations while recycling CO2. Energy storage is crucial for mass adoption of intermittent renewable energy generation. However, more optimization of the HFM reactor system is needed to generate higher product concentrations and productivity.
References:
- Ahmed A, Cateni B, Huhnke R, Lewis R. Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass and Bioenergy 2006;30:665-672.
- Kundiyana D, Huhnke R, Wilkins M. Syngas fermentation in a 100-L pilot scale fermentor: Design and process considerations. Journal of Bioscience and Bioengineering 2010;109:492-498.
- [Kundiyana D, Huhnke R, Madipatti P, Atiyeh HK, Wilkins M. Effect of cotton seed extract on synthesis gas fermentation using Clostridium strain P11. Bioresource Technology 2010; 101:9673-9680.
- Kundiyana D, Huhnke R, Wilkins M. Effect of nutrient limitation and two-stage continuous fermentor design on productivities during "Clostridium ragsdalei" syngas fermentation. Bioresource Technology 2011; 102:6058-6064.
- Kundiyana D, Wilkins M, Maddipati P, Huhnke R. Effect of temperature, pH and buffer on syngas fermentation using Clostridium strain P11. Bioresource Technology 2011; 102:5794-5799.
- Herzog HJ. What is the future for carbon capture and sequestration. Environmental science & technology 2001;35:148-153.
- Orgill, J.J., Atiyeh, H.K., Devarapalli, M., Phillips, J.R., Lewis, R.S., Huhnke, R.L. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresource Technology 2013; 133, 340-346.
Journal Articles on this Report : 1 Displayed | Download in RIS Format
| Other project views: | All 6 publications | 1 publications in selected types | All 1 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Ramachandriya K, Kundiyana D, Wilkins M, Terrill J, Atyeh H, Huhnmke R. Carbon dioxide conversion to fuels and chemicals using a hybrid green process. Applied Energy 2013;112:289-299. |
SU835171 (Final) SU834728 (Final) |
Exit |
Supplemental Keywords:
hexanol, biofuels, butanol, greenhouse gasProgress and Final Reports:
Original AbstractP3 Phase I:
BioWinol Technologies: A Hybrid Green Process for Biofuel Production | Final ReportThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.