Grantee Research Project Results
2015 Progress Report: Sustainable Catalytic Treatment of Waste Ion Exchange Brines for Reuse During Oxyanion Treatment in Drinking Water
EPA Grant Number: R835174Title: Sustainable Catalytic Treatment of Waste Ion Exchange Brines for Reuse During Oxyanion Treatment in Drinking Water
Investigators: Werth, Charles J , Strathmann, Timothy J.
Institution: University of Illinois Urbana-Champaign
EPA Project Officer: Packard, Benjamin H
Project Period: December 1, 2011 through November 30, 2014 (Extended to November 30, 2016)
Project Period Covered by this Report: December 1, 2014 through November 30,2015
Project Amount: $500,000
RFA: Research and Demonstration of Innovative Drinking Water Treatment Technologies in Small Systems (2011) RFA Text | Recipients Lists
Research Category: Drinking Water , Water
Objective:
The objectives of this work are to 1) identify palladium (Pd) catalyst formulations with sufficient activity to reduce different target oxyanions in brine solutions, 2) determine if catalyst activity can be maintained for extended periods of operations, and 3) assess the economic and environmental life cycle costs of hybrid ion exchange/catalyst treatment systems.
Progress Summary:
During the reporting period, significant progress was made with the pilot system. We designed, built and tested a recycle trickle bed pilot reactor (TBR). This was an iterative process, and required months of trouble shooting to stabilize liquid flow, evenly distribution flow across the catalyst beds, eliminate pump cavitation, and accurately measure bed pressure drop. The TBR system is much larger than our previous flow-through system, measuring roughly seven feet in height and two inches in diameter. For the last four months, we used the TBR with a 0.5 wt% Pd-0.05 wt% In/AC catalyst to test nitrate reduction using a synthetic brine for a range of different liquid and gas flow rates. We decided to use the 0.5 wt% Pd-0.05 wt% In/AC catalyst after testing 15 different commercial catalysts provided by Johnson Matthey, Inc. in a batch system. The selected catalyst is among the most active of those we tested on a Pd mass basis.
We are completing a manuscript that compares salt costs for traditional ion exchange with salt and catalyst costs for a hybrid ion exchange-catalyst system. The cost analysis is based on reaction rates we measured in batch and our original packed-bed column. We hope to submit this in the next month. Results from the TBR and new results from a pilot study of the TBR coupled with ion exchange, will be used to perform a more complete and updated cost assessment in 2016.
Task 1: Obtain and characterize brines from different water treatment plants.
This task is complete. We previously obtained and characterized three brines from water treatment plants (two in California, one in Minnesota). We have not observed significant difference in the performance of the catalysts treating these three brines. We will continue to use these brines in the flow-through column system.
Task 2: Test reduction rates and products in bench scale flow-through column system containing different combinations of catalysts using real brines.
A preferred catalyst was identified during the last reporting period and continued to be used during this reporting period. This catalyst is the 0.5 wt% Pd-0.05 wt% In/AC. During this reporting period, no flow-through studies were performed with real brine. Instead, we focused on designing a new trickle bed catalyst reactor in Task 4, and testing this with synthetic brine. Tests with real brine are planned for the upcoming reporting period.
Task 3: Test regeneration strategies for catalysts that become deactivated.
During the last reporting period, no work was performed on this task because we did not observe catalyst deactivation during the long-term flow-through column experiments. No further efforts are planned as part of this task.
Task 4: Build and test a pilot reactor for a small water treatment plant.
During the reporting period, we designed, built and tested a recycle TBR. This was an iterative process, and required months of trouble shooting to stabilize liquid flow, evenly distribution flow across the catalyst beds, eliminate pump cavitation, and accurately measure bed pressure drop. The TBR system is much larger than our previous flow-through system, measuring roughly seven feet in height and two inches in diameter. For the last four months, we used the TBR with the 0.5 wt% Pd-0.05 wt% In/AC catalyst to test nitrate reduction using a synthetic brine for a range of different liquid and gas flow rates. We decided to use the 0.5 wt% Pd-0.05 wt% In/AC catalyst after testing 15 different commercial catalysts provided by Johnson Matthey, Inc. in a batch system. The selected catalyst is among the most active of those we tested on a Pd mass basis.
Task 5: Evaluate environmental and economic benefits.
We are completing a manuscript that compares salt costs for traditional ion exchange with salt and catalyst costs for a hybrid ion exchange-catalyst system. The cost analysis is based on reaction rates we measured in batch and our original packed-bed column. We hope to submit this in the next month. Results from the TBR in Task 4, and new results from a pilot study of the TBR coupled with ion exchange, will be used to perform a more complete and updated cost assessment in 2016.
Results:
Task 1: Obtain and characterize brines from different water treatment plants.
This task was completed during the last reporting period.
Task 2: Test reduction rates and products in bench scale flow-through column system containing different combinations of catalysts using real brines.
No experiments were performed using real brines during this reporting period, and this task is complete.
Task 3: Test regeneration strategies for catalysts that become deactivated.
No work was performed on this task because we did not observe catalyst deactivation during our column studies. As a result, this task was modified and is now complete.
Task 4: Build and test pilot reactor for a small water treatment plant
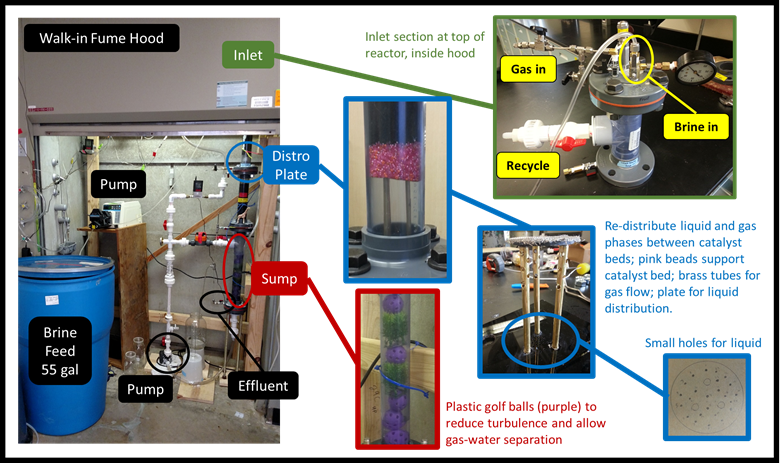
For the pilot system, we designed a new trickle bed reactor (TBR) to minimize mass transfer limitations of hydrogen from the gas to the liquid phase. Trickle bed reactors are commonly used for catalyst applications, though we found no literature regarding a trickle bed reactor for IX waste brine treatment. TBRs are commonly used in the hydrocarbon and fine chemical industries, frequently with non-aqueous fluids. Our design incorporated recycle, which was motivated by the physical space restrictions we have in the laboratory, and by the zero order rate constant for nitrate reduction we measured in batch. The design, construction and testing of the new reactor took about five months. During the process, we collaborated with a reactor design expert from Johnson Matthew Inc., whose insight proved invaluable for our design and trouble-shooting in order to bring the TBR online.
Designing the TBR was more challenging than designing the previous packed-bed reactors. One of the primary challenges was providing adequate space for gas and liquid flow through the reactor. This is a challenge at the reactor inlet distributor plate, and between catalyst beds at the mid-point distributor plate. Both hydrogen and carbon dioxide gas are required; the former is the electron donor and the latter is used for pH control. It took multiple attempts to find the right combination of gas and liquid flow port areas and flow port numbers to accommodate the range of liquid and gas flows desired. As shown in Figure 1, the distributor plate is comprised primarily of three parts: 1) the black plate on the bottom, which distributes liquid onto the bed below; 2) the vertical brass tubes, which have holes to provide a flow path for gas between the catalyst beds; and 3) the washer/screen on the top, which supports the catalyst bed above. Below the distributor plate, the liquid and gas mix and flow through the catalyst bed.
The other primary challenge is related to our brine matrix. Brine was more “frothy” in the sump than clean water, which created a siphon effect in the reactor, promoted pump cavitation, and prevented continuous operation. After testing several ideas, we incorporated plastic golf balls (see picture outline in red) to reduce turbulence and enable gas-liquid separation prior to the effluent port. Brine also impacted the operations of the TBR by reacting with components. It reacted with steel reactor components, including a liquid flow meter and a catalyst support plate used above the distributor plate. This recently caused in interruption in TBR options. These valuable lessons are allowing us to improve TBR design and improve reactor operation.
Figure 1: Pictures of the trickle bed reactor with close-up details of selected components.
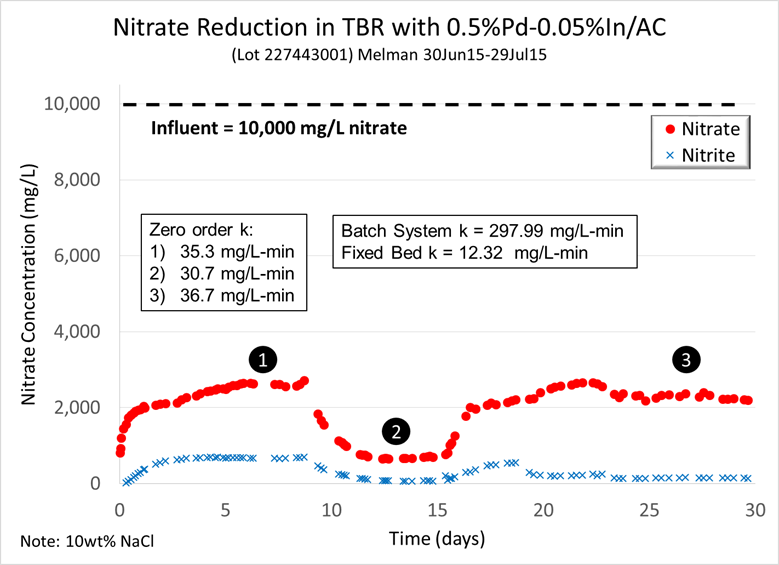
After completing the start-up tests, we ran a 30-day experiment with synthetic brine and the 0.5 wt% Pd-0.05 wt% In/AC catalyst. We used a 10 wt% NaCl and 10,000 mg/L nitrate brine. The effluent nitrate concentration during the experiment is shown in Figure 2. The level of nitrate reduction is very consistent for the same TBR inlet conditions. More importantly, the reduction activity in the TBR is at least 250% greater than in our original packed-bed reactor (30 mg/L-min versus 12.32 mg/L-min). However, the improved rates are still about 10% of the intrinsic catalyst activity measured in a batch reactor (~300 mg/L-min). This indicates hydrogen mass transfer still limited the overall reaction rate in the TBR, and motivated us to evaluate different liquid and gas flow rates to minimize hydrogen mass transfer limitations.
Figure 2: Results from the first TBR experiment, 30 days long.
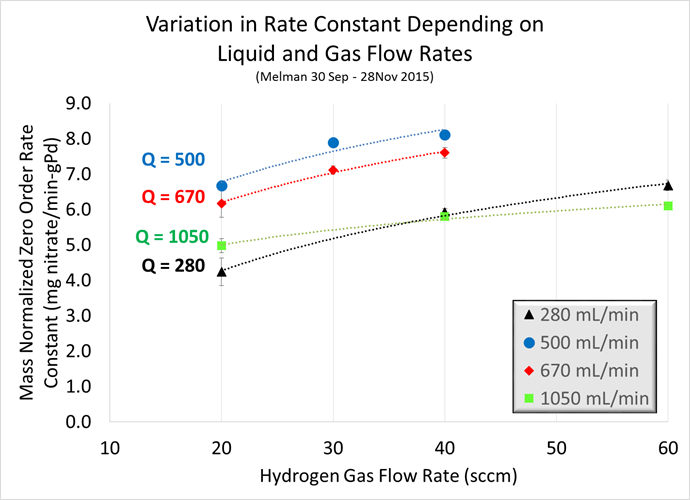
In the second TBR experiment, we tested 12 different flow conditions across a range of liquid and gas flow rates. Table 1 lists the conditions tested. For this experiment, we used a synthetic brine containing 10 wt% NaCl and 14,000 mg/L. The objective was to evaluate different combinations of liquid and gas flow rates to maximize nitrate reduction activity by minimizing hydrogen mass transfer limitations. The recycle rates varied between 280 and 1050 mL/min, and the hydrogen gas flow rates varied between 20 and 60 sccm. Carbon dioxide gas was varied only slightly to maintain pH between 7.0 and 8.0, which previous results have demonstrated promotes high selectivity (70-80%) for dinitrogen gas.
Table 1: Conditions tested during 58 day TBR experiment
| Liquid Flow Rate | 280 mL/min | 500 mL/min | 670 mL/min | 1050 mL/min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen Gas Flow Rate (sccm) | 20 | 40 | 60 | 20 | 30 | 40 | 20 | 30 | 40 | 20 | 40 | 60 |
| Carbon Dioxide Gas Flow Rate (sccm) | 10 | 13.5 | 14 | 10 | 13 | 13.5 | 10 | 13 | 13.5 | 10 | 13.5 | 14 |
The variation in mass normalized zero order rate constant as a function of liquid and gas flow rates is shown in Figure 3. Each data point is one condition tested; each line on the graph represents the results from one recycle rate tested at three different gas flow rates. For example, the blue circles show a recycle rate of 500 mL/min tested with hydrogen gas flow rates of 20, 30 and 40 sccm. In all cases, catalytic activity increased with higher gas flow, which is expected. The variation in activity as a function of recycle rate is not linear. The lowest activity results from the highest and lowest recycle rates (1050 and 280, respectively), and the highest activity results from intermediate recycle rates (500 and 670). These results suggest that the optimal conditions for TBR operations are with the intermediate liquid and high gas flow rates tested.
Figure 3: Results from 58 day TBR experiment with various liquid and gas flow rates.
We are still evaluating higher and lower liquid flow rates in the TBR, and hope to complete this in the next two months. For the gas and liquid flow conditions that result in the greatest activity (and lowest hydrogen mass transfer limitations), we also plan to evaluate TBR performance with a diluted catalyst, i.e., pack catalyst bed with a mixture of supported catalyst and inert support. This should also reduce hydrogen mass transfer limitations for the same flow conditions. We are developing a numerical model to calculate hydrogen mass transfer rates for our experiments. We will compare these rates to literature values to see if we are missing some obvious improvement in our TBR design.
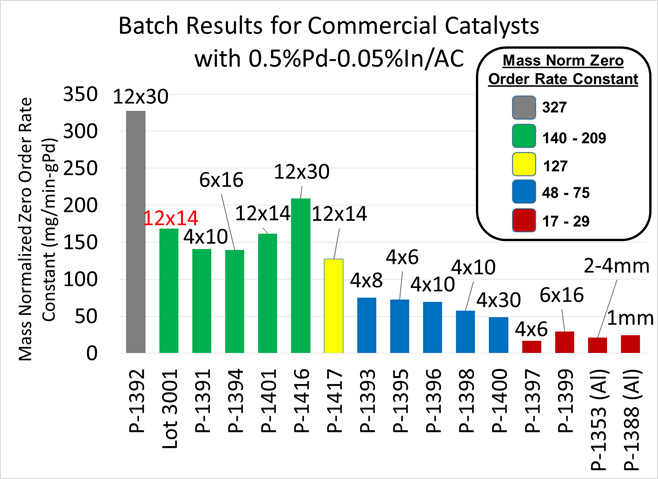
Lastly, in coordination with Calgon Carbon and Johnson Matthey, Inc., we conducted a batch study to test 15 commercial catalyst supports to explore the possibility of increasing catalyst activity through the use of a different size support, i.e., the size of the carbon particle holding the metal catalyst. A smaller catalyst support should increase activity by minimizing boundary layer and intra-particle mass transfer limitations, and possibly by increasing the surface area to volume ratio of hydrogen gas passing through the reactor. A comparison of the results from batch studies can be seen in Figure 4. The results fell into different groups of activity, which are noted by colors. The labels above each bar denote the size of the support in US mesh sizes, i.e., 12x14 = 12 mesh by 14 mesh. The current 0.5 wt% Pd-0.05 wt% In/AC catalyst we have been using for the last 2 years is the first green bar on the left, “Lot 3001,” and has a red “12x14” label above the bar. All other bars are new catalysts we tested and are all manufactured on the commercial scale.
Figure 4: Comparison of the rate constants from commercial catalysts.
The main outcome of these experiments was to identify an issue with variability in the carbon. For example, there are two 12 x 30 catalysts shown on the plot (P-1392 in grey and P-1416 in green), but their rate constants were significantly different. While the activity of P-1392 is extremely promising, the lower activity of P-1416 raises questions about the overall reliability of the 12x30 support. Similarly, of the 4x10 supports tested, the activity of P-1391 was much higher than both P-1396 and P-1398. Two alumina support catalysts were tested (P-1353 and P-1388) but their rates were very low, as expected based on previous results. Alumina is an ineffective catalyst for nitrate reduction in brine conditions.
Task 5: Evaluate environmental and economic benefits.
We previously developed a numerical model to simulate ion exchange. We recently used a new approach to fit model parameters, and were able to produce results that more accurately represent multi-cycle experimental data. These model improvements are important to support our hybrid system analyses, which are described in a draft manuscript.
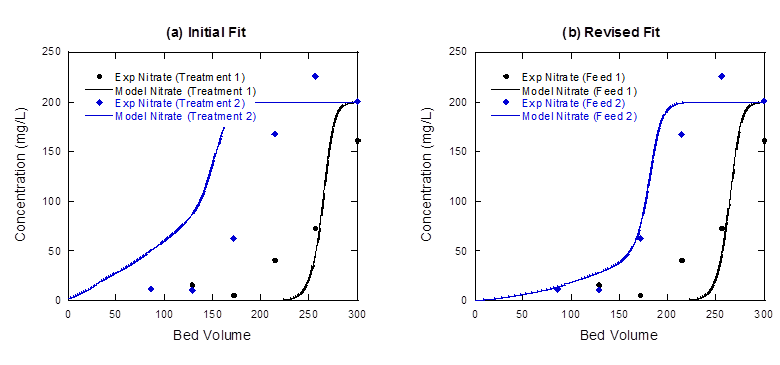
For our new model fitting approach, we simulated two cycles of ion exchange treatment and regeneration data, the most challenging experimental conditions we evaluated. We validated the new model parameters by predicting the performance of additional two cycle experiments that were not used in parameter calibration. The improvement of our model fit to nitrate breakthrough curves during treatment can be seen in Figure 5 below. In Figure 5a, the initial parameters did not predict nitrate breakthrough well during the second cycle, as indicated by the gap between the model fit (blue solid line) and experimental data (blue diamonds). In Figure 5b, the new model parameters are used and the prediction for nitrate breakthrough during the second treatment cycle is much more accurate.
Figure 5: Nitrate breakthrough curves during two-cycle ion exchange experiments, and (a) model predictions using the initial best-fit regeneration parameters, and (b) the revised best fit model parameters.
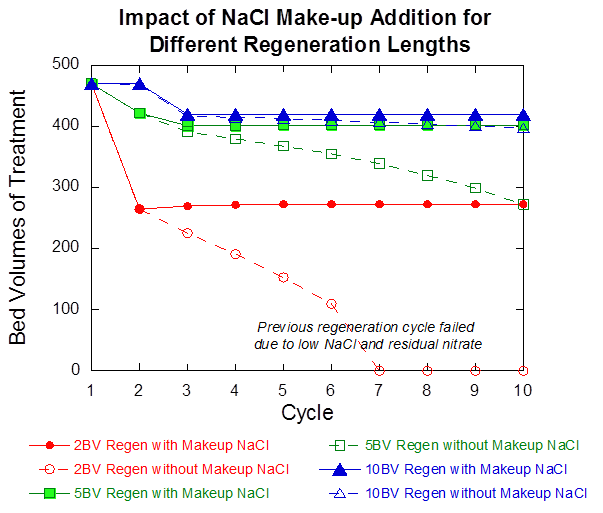
After refining the model parameters, we evaluated the impact of makeup NaCl on the hybrid system during 10 cycles of treatment and regeneration. The results are shown in Figure 6. Not adding makeup NaCl to the brine limited the number of times it could be reused before failing to regenerate the resin, due to the loss in NaCl during each cycle. Longer regeneration time extends the number of brine reuse opportunities, with 2 BV being reused five times, and 5 BV and 10 BV being reused for all ten cycles, but to different levels of success. The increased reuse capability without makeup NaCl between 2 BV and 10 BV results because of the larger initial volume of brine used and the relatively small fraction of total chloride lost (3.2% for 10 BV versus 11.5% for 2 BV). Hence, makeup salt addition is very important for all regeneration BV below 10 for up to 10 cycles of brine reuse. It also is interesting to note that makeup NaCl has only a marginal effect when using 10 BV for regeneration, due to the small fraction of chloride lost during each regeneration cycle
Figure 6: Impact of the number of regeneration BVs and NaCl makeup addition on the number of BV until breakthrough during the next treatment cycle. Solid lines indicate scenarios in which makeup NaCl is added; dashed lines do not include makeup NaCl.
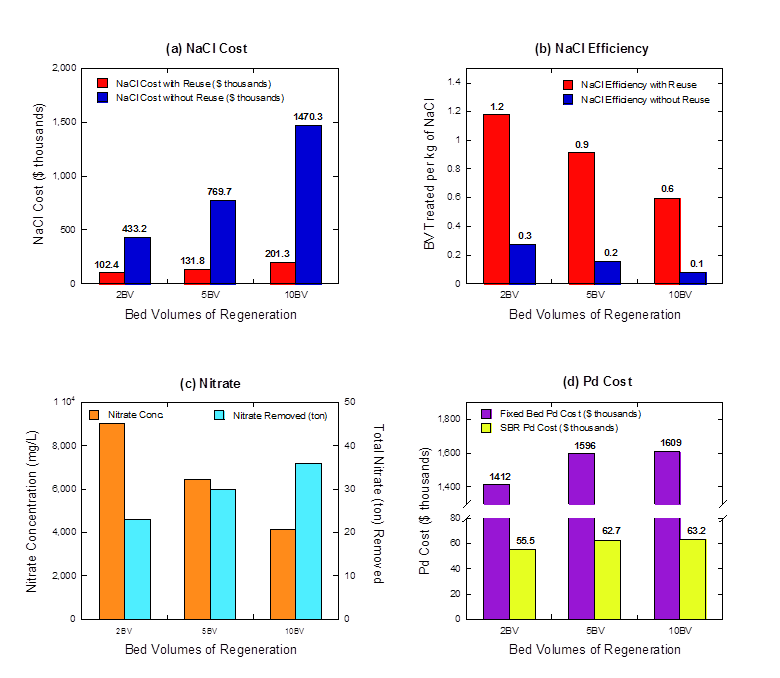
Finally, we evaluated the effects of the number of BV used for regeneration on the treatment of one billion gallons of water with the hybrid system (Figure 7), and compared this to results for conventional IX treatment without brine reuse. Calculations were based on multi-cycle model simulations and IX system information from the Vale, OR demonstration plant study (0.5 MGD), which had a single-pass IX configuration that treated water at 540 gpm. Catalyst requirements for denitrifying the simulated waste brine to a level of 1,500 mg/L NO3- were estimated using the zero-order rate constants from the fixed-bed reactor (2.24 mg NO3- gPd-1 min-1) and sequencing batch reactor (57 mg NO3- gPd-1min-1), which both used the same 0.5 wt% Pd-0.05 wt% In/C catalyst.
The cost of NaCl with and without brine reuse with respect to different regeneration lengths is shown in Figure 7a. When brine is not reused, a 10 BV regeneration strategy requires more than three times as much NaCl as a 2 BV regeneration strategy (2591 ton vs 8793 ton). Alternately, when brine is reused, the quantities are much lower and the difference is much smaller (612 ton vs 1204 ton). Thus, by switching from 2 BV regeneration without brine reuse to 10 BV regeneration with brine reuse, the plant operator would see a 53% decrease in total NaCl required and 150% increase in treatment length each cycle (274 BV to 420 BV). The efficiency of NaCl use as a ratio of BV of water treated per kilogram of NaCl used is shown in Figure 7b. The results show a clear benefit to brine reuse (red), which leads to between 4.2 and 7.3 times more water treated per mass of NaCl consumed. The results also show a clear benefit in salt cost to regenerating with only 2 BV, compared to 5 or 10 BV.
The average nitrate concentration in the waste brine prior to catalytic treatment (orange), as well as the total mass of nitrate removed from the waste brine to treat 1 billion gallons (blue), are shown in Figure 7c. Nitrate concentrations in the waste brine, as well as the volume of waste brine to be treated and time available for treatment, are all impacted by the regeneration length. Together, these variables impact one of the most expensive parts of the hybrid system capital costs, which is palladium metal. These costs are shown in Figure 7d for 2, 5, and 10 BV of regeneration. A 2 BV regeneration produces a smaller volume of waste brine with a higher nitrate concentration compared to a 5 or 10 BV regeneration. A smaller volume requires less catalyst for treatment, while a higher nitrate concentration requires more catalyst. The former dominates overall Pd metal costs, so Pd metal costs decrease with decreasing BV of regeneration
Figure 7: Analysis of hybrid system requirements to treat one billion gallons of water depending using different BV for regeneration between each nitrate treatment cycle with and without brine reuse. (a) Cost of NaCl required. (b) Efficiency of NaCl use – defined as BV water treated per kilogram of NaCl required. (c) Nitrate concentration in the waste brine before catalytic reduction and total mass of nitrate removed from waste brine to treat 1 billion gallons. Higher concentrations require more reduction to reach the desired 1,500 mg/L level, which impacts the catalyst required. (d) Catalyst cost as a function of regeneration BV for fixed bed and sequencing batch reactor configurations.
Catalyst costs are also related to catalyst activity level. The anticipated costs of Pd required to reduce nitrate in the waste brine to 1,500 mg/L nitrate using the mass normalized zero order rate constant for both the fixed bed system and sequencing batch reactor are shown in Figure 7d. As discussed above, catalyst activity in the fixed bed is relatively low compared to the batch system due to mass transfer limitations, resulting in prohibitively high palladium costs (purple), i.e., costs that far exceed those associated with reduced salt usage. However, when the catalyst activity from the SBR is used to calculate Pd requirements, costs are much lower (yellow). Considering the SBR activity, a 2 BV regeneration leads to nitrate concentrations around 9,000 mg/L and a palladium cost of $55.5k; whereas 10 BV regeneration leads to nitrate concentrations around 4,100 mg/L and a palladium cost of about $63.2k. These costs are 50% or lower than those associated with cost savings from brine reuse when treating 1 billion gallons of source water, and highlight the promise of the hybrid system.
Future Activities:
During the next reporting period, our primary efforts will focus on conducting more trickle bed reactor experiments and evaluating the life cycle and economic benefits of the hybrid system. We also will conduct TBR experiments with real brine.
Journal Articles on this Report : 8 Displayed | Download in RIS Format
| Other project views: | All 57 publications | 15 publications in selected types | All 15 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Bergquist AM, Choe JK, Strathmann TJ, Werth CJ. Evaluation of a hybrid ion exchange-catalyst treatment technology for nitrate removal from drinking water. Water Research 2016;96:177-187. |
R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
|
|
Choe JK, Mehnert MH, Guest JS, Strathmann TJ, Werth CJ. Comparative assessment of the environmental sustainability of existing and emerging perchlorate treatment technologies for drinking water. Environmental Science & Technology 2013;47(9):4644-4652. |
R835174 (2012) R835174 (2013) R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
|
|
Choe JK, Boyanov MI, Liu J, Kemner KM, Werth CJ, Strathmann TJ. X-ray spectroscopic characterization of immobilized rhenium species in hydrated rhenium–palladium bimetallic catalysts used for perchlorate water treatment. The Journal of Physical Chemistry C 2014;118(22):11666-11676. |
R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
|
|
Choe JK, Bergquist AM, Jeong S, Guest JS, Werth CJ, Strathmann TJ. Performance and life cycle environmental benefits of recycling spent ion exchange brines by catalytic treatment of nitrate. Water Research 2015;80:267-280. |
R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
|
|
Liu J, Choe JK, Sasnow Z, Werth CJ, Strathmann TJ. Application of a Re–Pd bimetallic catalyst for treatment of perchlorate in waste ion-exchange regenerant brine. Water Research 2013:47(1):91-101. |
R835174 (2012) R835174 (2013) R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
|
|
Liu J, Choe JK, Wang Y, Shapley JR, Werth CJ, Strathman TJ. Bioinspired complex-nanoparticle hybrid catalyst system for aqueous perchlorate reduction:rhenium speciation and its influence on catalyst activity. ACS Catalysis 2015;5(2):511-522. |
R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
|
|
Liu J, Chen X, Wang Y, Strathmann TJ, Werth CJ. Mechanism and mitigation of the decomposition of an oxorhenium complex-based heterogeneous catalyst for perchlorate reduction in water. Environmental Science & Technology 2015;49(21):12932-12940. |
R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
|
|
Zhang R, Shuai D, Guy KA, Shapley JR, Strathmann TJ, Werth CJ. Elucidation of nitrate reduction mechanisms on a Pd-In bimetallic catalyst using isotope labeled nitrogen species. ChemCatChem 2013;5(1):313-321. |
R835174 (2012) R835174 (2013) R835174 (2014) R835174 (2015) R835174 (Final) |
Exit Exit Exit |
Supplemental Keywords:
Nitrate, perchlorate, ion exchange, brine reuse, catalytic reduction, palladium, indium, rhenium, toxics, innovative technology, cost-benefit, integrated assessment.Progress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.