Grantee Research Project Results
Final Report: Novel 'Greener' Routes to Halogen-Free Flame Retardant Materials
EPA Grant Number: SU835071Title: Novel 'Greener' Routes to Halogen-Free Flame Retardant Materials
Investigators: Nagarajan, Ramaswamy , Kumar, Jayant , Ravichandran, Sethumadhavan , Bouldin, Ryan , Kiratitanavit, Weeradech , Xia, Zhiyu
Institution: University of Massachusetts - Lowell
EPA Project Officer: Page, Angela
Phase: II
Project Period: August 15, 2011 through August 14, 2013 (Extended to August 14, 2014)
Project Amount: $75,000
RFA: P3 Awards: A National Student Design Competition for Sustainability Focusing on People, Prosperity and the Planet - Phase 2 (2011) Recipients Lists
Research Category: Pollution Prevention/Sustainable Development , P3 Awards , P3 Challenge Area - Chemical Safety , Sustainable and Healthy Communities
Objective:
Polymeric materials have become an indispensable part of human life in commodity applications like electronic components, textile fabric, upholstery, etc. However, these materials have one major drawback in the intrinsic flammability. To reduce the rate of flame propagation, additives called flame retardants (FR) are usually compounded into plastics. More than one-third of FR additives used currently globally are halogenated (brominated/chlorinated) compounds. These compounds have been reported to be toxic to humans and environmentally persistent. Recently many countries have regulated their use. This research was directed towards the development of a new class of non-halogenated, non-toxic polymeric FR based on polyphenols. During phase-I of this project, the P3 student team has developed a class of thermally stable polymers, with a polyphenol structural backbone. Specifically, the team identified and utilized a renewable, cost-effective industrial byproduct called cardanol as starting material for the synthesis of polyphenols for possible use as alternative FR additives. The procedure developed for the synthesis of these materials utilizes environmentally friendly enzymatic or biomimetic catalysts and the reaction were carried out in predominantly aqueous reaction media. The polyphenols (specifically polycardanol and polydeoxybenzoins) developed in Phase-I had low heat release capacities and high char forming capability suitable for FR additives.
Objective for phase II:
- Scale-up of synthesis, specifically polycardanol developed in phase-I
- Incorporation/blending of polycardanol with polyolefins and evaluation of thermal stability and flame retardancy using TGA and PCFC
- Development of new FR, combining polyphenols with organically modified clays and metal oxides for efficient gas phase and condensed phase action
- Study of degradation kinetics of polyphenols using combination of techniques such as TGA and FTIR
- Study of rheology of polycardanol blended with polyolefin
- Toxicity evaluation of polycardan
Summary/Accomplishments (Outputs/Outcomes):
Reaction scale up: Commercial success of any FR additive as potential replacement for halogenated FR is only possible if the raw materials are easily available at a low cost and the reaction is scalable to large volumes at a reasonable cost. Polycardanol was synthesized in the scale of 10-20 grams per batch in 5 liter lab-scale reactor. A maximum yield of 87% was attainable for reactions with catalyst/monomer ratio of 2. After purification and drying, polycardanol was ground into fine particles using an ultra centrifugal mill purchased during Phase - II. The final particle size of polycardanol was around 30 micrometer where as the particle size of commercial halogenated FR, decabromodiphenylether (DBDPE) and hexabromocyclododecane (HBCD) was around 3-5 micron.
Blending of polycardanol with polyolefins: Polycardanol was compounded with polyolefins [Polypropylene (PP) and Low density polyethylene (LDPE)] using a CW Brabender type 6 mixer. Blends containing 0, 1, 5, 10 and 15 by weight % of polycardanol were prepared. Thermal characterization was carried out on these compounded samples.
Thermogravimetric analysis (TGA): From the TGA results it was obvious that, virgin PP and PE undergo thermal degradation under nitrogen atmosphere at around 463oC and 473oC. When polycardanol is added into these systems, there was an appreciable increase in decomposition temperature (Td) and a marginal increase in char forming capability of these blends. In case of LDPE, there is up to a 30oC increase in Td and 3.2% char yield upon 15 weight % addition of polycardanol. The increase in Td (~35oC) and char yield (4.2%) for PP-polycardanol blends was slightly higher than that of LDPE-polycardanol blends. In both cases, the beneficial effects start plateauing after 10 by weight % additive loading.
Pyrolysis Combustion Flow Calorimetry (PCFC): PCFC or micro-scale combustion calorimetry was used to calculate total hear release (THR) and Heat release capacity (HRC) values of the blends. THR and HRC are reasonable predictors of polymer flammability.
Total Heat Release: As seen in Table 1. neat LDPE and PP have THR at 45.7 and 40.6 KJ/g, respectively. Polycardanol/polyolefin blends show a 9% and 7% reduction in THR for LDPE (41 KJ/g) and PP (37.8 KJ/g), respectively at 10% loading of polycardanol. This reduction in THR is comparable to previously reported values for THR decrease in blends of polyolefins with phosphorous-based flame retardants. However, at this point, the type and nature of the condensed phase reactions in polyolefin/polycardanol blends that lead to THR increase is not very well understood.
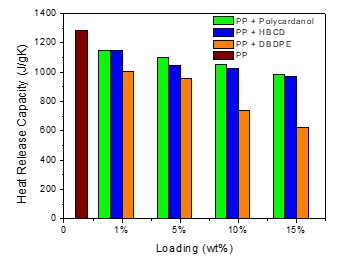
Figure 1. Comparison of HRC of polycardanol/PP, DBDPE/PP and HBCD/PP blends
| Polymer | THR (KJ/g) | HRC (J/g-K) |
| LDPE | 45.7 | 1273 |
| LDPE + 1 PC | 44.9 | 1173 |
| LDPE + 5 PC | 43.0 | 1145 |
| LDPE + 10 PC | 41.0 | 1115 |
| LDPE + 15 PC | 41.6 | 1149 |
| PP | 40.6 | 1288 |
| PP + 1 PC | 42.5 | 1151 |
| PP + 5 PC | 39.7 | 1100 |
| PP + 10 PC | 39.7 | 1056 |
| PP + 15 PC | 37.8 | 986 |
Table 1. PCFC results of polycardanol/polyolefin blends
Heat Release Capacity: In PP/polycardanol and LDPE/polycardanol blends, maximum degradation of the virgin material coincides with the temperature at the heat release rate (pHRR) peaks, which is typically at 471oC and 480oC, respectively. However, with increase in polycardanol concentration, there is a decrease in pHRR as well as HRC as seen in Table 1 and Figure 1. It is hypothesized that the polycardanol strongly influences the decomposition of polyolefins in the gas and/or condensed phase increasing the peak degradation temperature. Polycardanol also delays the complete decomposition of polyolefins.
The HRC of polycardanol PP blends were compared with those of blends containing decabromodiphenylether (DBDPE) and hexabromocyclododecane (HBCD) at various loading levels. DBDPE, is one of the most efficient halogenated FR. There is up to a 50% reduction in HRC with respect to virgin PP at 15% loading. HBCD, which is a moderate FR for PP is often used in combination with metal hydroxide additives for synergistic action. In our study, HBCD showed a 24% reduction in HRC at 15% loading. There is a 23% reduction in HRC with in PP-polycardanol blends when the loading level of polycardanol is 15% by weight. It is interesting to note that polycardanol shows promise in terms of improving the thermal stability of PP (increasing the thermal-decomposition temperature).
Degradation kinetic of polyphenols
Combination of thermogravimetric analysis and Fourier-transform infrared spectroscopy (TGA-FTIR) provides a very useful tool for the possible determination of the degradation pathway of polymers, and the influence of FR additives on the degradation pathway.
The thermal degradation of polycardanol is a multistage process. At the first stage carbon dioxide is released mainly starting from 6 minutes (140℃). From 20 minutes (420℃), alkanes, aromatics, and methane start to be released. At the second stage volatile alkanes are released due to the degradation of the alkyl chains in cardanol. Aromatics are also released from the degradation of polycardanol. The release of methane is mainly from the combination of methyl radical and hydrogen radical. In the last stage carbon monoxide is generated due to the decomposition of phenoxy radicals. The remaining material is converted mainly to graphitic char. When comparing the Grams-Schmidt FTIR curves of polypropylene with polypropylene/cardanol blends it is evident that the increased concentration of carbon dioxide and carbon monoxide in the blends are mainly due from the presence of polycardanol. PCFC test calculates the heat release capacity based on the consumption rate of oxygen that oxidizes the volatiles in the combustor. The releases of carbon dioxide and char formation contribute to the low heat release capacity of the blends.
Synthesis of synergistic Clay-polyphenol combinations
Nanoclay when blended into plastics are known to decrease the heat release rate and HRC of the blends. However they are not very effective when used alone. Synergistic combination of nanoclay with other types of FR (halogenated or phosphorus containing varieties) is known to be more efficient even at low loading levels. During Phase-II it was demonstrated that this type of synergy could also be obtained by physical adsorption of phenol on organic nanoclays followed by subsequent polymerization. The nanoclay coated with polyphenol exhibits synergistic reduction in HRC when blends into PP.
Toxicity evaluation of polyphenols
One of the goals of the project was to explore the possibility of developing non-toxic alternatives to toxic halogenated FR. Although the monomer (cardanol) has been reported to be a skin irritant but biodegradable (96% of original weight degraded within 28 days) the toxicity of the polymer of cardanol had to be evaluated. The molecular structure of polymer is different from that of the monomer and therefore the toxicity profile can be quite different. To explore the possibility of using polycardanol as possible non-halogenated FR for plastic, the toxicity of this FR was evaluated in accordance to the Organization for Economic Co-Operation and Development (OECD) 425: guidelines for the testing of chemicals, Acute Oral Toxicity (up and down procedure) by Toxicon Corporation. The result from this test provided the LD50 value.
Five female Sprague Dawley rats were selected by Toxicon as test animals. Polycardanol at three different weights (0.1, 0.3 and 1 g) were mixed with cotton seed oil (CSO) at three different doses (175, 550 and 2000 mg/kg, the unit -mg- is for the weight of polycardanol and the unit -kg- is for the body weight of test animal). Then the mixture was fed to the test animals (175 and 550 mg/kg doses were used on animal #1 and #2 respectively and the dose at 2000 mg/kg was used on animal #3 - #5). After feeding, the test animals were observed for their response in first 4 hours and subsequently daily for the following 14 days. For the purpose of evaluation of acute toxicity, all abnormalities (including behavioral and clinical), gross lesion, bodyweight changes, effects on mortality and any other toxic effects were observed, measured and recorded. The LD50 value and the confidence intervals were calculated and determined by using the statistical computer program (AOT425StatPgm) developed by the EPA. It was found that the weight of all test animals increased and no unusual behaviors were observed during the test period. None of the animals dosed at three different levels died during the tests. The LD50 of polycardanol was estimated to be greater than 2000 mg/kg.
Conclusions:
A new class of non-halogenated flame retardant, char forming additive based on polyphenols was successfully synthesized, characterized and tested by blending into plastics. In the phase II of the EPA funded P3 project, the team scaled up (to several hundred gram scale) the synthesis of a polymer of cardanol. A low cost, bio-derived phenol namely cardanol was polymerized to yield polycarandol with improved thermal stability and reasonable compatibility with polypropylene. Polycardanol could be dispersed into polyolefins such as PP and the blends, exhibited higher degradation temperatures, lower HRC and higher char yield. The performance of this polyphenol compares favorably with some commercially available halogenated FR (such as DBDPE and HBCD) purely from a heat release capacity (HRC) perspective. TGA-FTIR evolved gas analysis studies indicated that the release of carbon dioxide and formation of char contributes to the lower HRC of polycardanol and PP-polycardanol blends. Polycardanol seems to retard/delay the release of hydrocarbon based degradation compounds from polypropylene upon heating. Polycardanol appears to be non-toxic based on toxicity studies carried out in accordance to OECD 425 method. Polycardanol has an LD50 greater than 2000 mg/kg. This material can be blended with polyolefins to work as char forming FR additive comparable in performance to certain types of commercial halogenated FR.
Journal Articles on this Report : 1 Displayed | Download in RIS Format
| Other project views: | All 6 publications | 1 publications in selected types | All 1 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Kiratitanavit W, Ravichandran S, Xia Z, Kumar J, Nagarajan R. Thermally stable polymers of cardanol as char-forming additives for polypropylene. Journal of Renewable Materials 2013;1(4):289-301. |
SU835071 (Final) SU834738 (Final) |
Exit |
Supplemental Keywords:
Non-halogenated flame-retardants, renewable feedstock, green chemistry, chemical catalyst, benign method, cardanolProgress and Final Reports:
Original AbstractP3 Phase I:
Novel ‘Greener’ Routes to Halogen-free Flame Retardant Materials | Final ReportThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.