Grantee Research Project Results
2014 Progress Report: Endotoxin Exposure and Asthma in Children
EPA Grant Number: R834515Center: Denver Childrens Environmental Health Center - Environmental Determinants of Airway Disease in Children
Center Director: Guo, Yanbing
Title: Endotoxin Exposure and Asthma in Children
Investigators: Schwartz, David A. , Covar, Ronina A , Litonjua, Augusto A. , Liu, Andrew H. , Murphy, Amy J , Strand, Mathew J. , Van Dyke, Michael V. , White, Carl W. , Martyny, John W. , Forssen, Anna , Dakhama, Azzeddine , Yang, Ivana , Yang, Jing , Loader, Joan , Sordillo, Joanne , Oakes, Judy , Correll, Kelly , Gabehart, Kelsa , Rabinovitch, Nathan , Szefler, Stanley
Current Investigators: Schwartz, David A. , Covar, Ronina A , Litonjua, Augusto A. , Liu, Andrew H. , Murphy, Amy J , Strand, Mathew J. , Van Dyke, Michael V. , Martyny, John W. , Rabinovitch, Nathan
Institution: National Jewish Health , Tri-County Health Department, CO , Channing Laboratory , Harvard University
Current Institution: National Jewish Health
EPA Project Officer: Hahn, Intaek
Project Period: June 22, 2010 through June 21, 2015 (Extended to June 21, 2017)
Project Period Covered by this Report: June 22, 2014 through June 21,2015
Project Amount: $1,897,209
RFA: Children's Environmental Health and Disease Prevention Research Centers (with NIEHS) (2009) RFA Text | Recipients Lists
Research Category: Children's Health , Human Health
Objective:
Project 1 – ENVIRONMENTAL EXPOSURE AND ASTHMA IN CHILDREN
We hypothesize that higher levels of endotoxin exposure cause persistent, problematic asthma and that key environmental (ozone and allergens) and genetic modifiers (endotoxin receptor polymorphisms) contribute to endotoxin susceptibility and pathological asthmatic responses. We are studying these endotoxin-induced airway conditions in children through three complementary clinical investigations. First, we are capitalizing on an ancillary study of an NIH-sponsored multi-center cohort of children with asthma (Childhood Asthma Management Program (CAMP)), which has tracked asthma severity for more than a decade, to determine if endotoxin exposure, modified by genetics and environment, is associated with greater disease severity and persistence.
Second, we have planned a panel study of children with asthma to investigate whether endotoxin exposure, modified by environment, is associated with inflamed airways and elevated TLR expression on airway macrophages. Clinically, these inflammatory responses could drive poor asthma control and exacerbations.
Finally, we have taken advantage of a Housing and Urban Development (HUD)-sponsored inner-city home intervention study to determine if a home environment intervention will reduce home endotoxin levels and improve asthma. This combination of studies is expected to provide an understanding of how endotoxin interacts with other potentially toxic exposures in the susceptible host to cause persistent, problematic asthma. These studies will help us to determine the levels of endotoxin exposure that are likely to be problematic for children with asthma, and to develop environmental educational and intervention programs to improve health outcomes.
Project 2 – ENDOTOXIN DETERMINANTS OF EARLY HOST RESPONSE TO RESPIRATORY SYNCYTIAL VIRUS (RSV)
The pollutant ozone is suspected to play a significant role in the development and exacerbation of reactive airway diseases such as asthma. Ozone exposure may alter lung development and growth, especially in the early postnatal development phase, resulting in increased susceptibility to airway obstruction upon subsequent viral infection and allergen exposure. Ozone exposure also can influence the innate immune response by increasing Tolllike receptor (TLR)‐4 expression, thereby increasing airway responsiveness to bacterial endotoxin (LPS). LPS also is known to mature dendritic cells, the primary immune cell subset that initiates T-cell differentiation and directs the adaptive response. The overall hypothesis of this project is that ozone exposure, in the early postnatal phase, alters lung development and modifies the host immune response to early life viral infection and allergen exposure, thereby contributing to the development of reactive airway disease. In the presence of LPS, however, lung development will be sustained and the host immune response will mature and protect the newborn against the development of altered airway responses to viral infection and allergen exposure.
Specific Aim 1: To define the influence of ozone exposure on TLR expression and airway structure and function.
Specific Aim 2: To define the influence of ozone exposure on airway responsiveness to respiratory syncytial virus (RSV) and allergen.
Specific Aim 3: To determine if and how LPS can modify airway responsiveness to RSV infection and allergen, following postnatal exposure to ozone.
Project 3 – ENDOTOXIN DETERMINANTS OF EARLY HOST DEFENSE
The overall goal of this project is to understand how and why air pollution alters lung host defense. While the proposed research is focused on mice, we believe that the discoveries we make in mice will prove to be relevant to basic mechanisms of lung host defense in children. In fact, our findings in mice will be tested in Project 1 of this program. The environmental, clinical, and biological significance of this project is supported by the following observations. First, air pollution accounts for substantial morbidity and mortality throughout the world, including lung infections and preventable deaths in children. Second, endotoxin is ubiquitous in the environment and is associated with the development and progression of asthma and other forms of airway disease. However, the relationship between endotoxin and asthma is not simple because early childhood exposure to endotoxin, at least in certain populations, appears to protect children from developing asthma and atopy. Air pollution is contaminated with endotoxin, so this pathogen associated molecular pattern (PAMP) or other PAMPs may play a role in the pathophysiology of air pollution. Third, the ability of the host to respond to lipopolysaccharide (LPS; a specific form of endotoxin) and other PAMPs is highly variable in mice and humans, yet polymorphic host defense genes only account for a portion of this variable response. Fourth, innate immunity provides a first line of host defense against microbial pathogens that is conserved over a wide variety of species from flies to mammals. Indeed, innate immune signaling mechanisms in mice are almost identical to those in humans. Finally, the innate immune system is biologically dynamic and is responsive to both ozone and PAMPs. We recently have found that the expression of innate immune receptors on macrophages can be enhanced by ozone or PAMPs. Moreover, others have reported that some innate immune cells avoid excessive inflammation by selectively downregulating proinflammatory genes while continuing to transcribe antimicrobial genes. Thus, the overall hypothesis of this project is that the expression of toll-like receptors (TLRs) in the lung is influenced by environmental (ozone and/or PAMPs) and genetic factors, and the dynamic expression of TLRs has profound effects on lung host defense and, consequently, the development of lung infections and allergic airway disease.
Progress Summary:
During the fifth year of this project on environmental asthma, substantial progress has taken place. All three independent projects and the three Cores have made excellent progress and have met all of their goals. The overall structure and necessary interactions within the Program is established, productive, and is insuring the continued success of the Program. Center investigators meet at least every month and scientific discussions and scientific collaborations between the investigators are naturally evolving. We are actively engaged in considering scientific options for our competitive renewal.
Project 1–ENVIRONMENTAL EXPOSURE AND ASTHMA IN CHILDREN
As proposed, three complementary studies address the aims and hypotheses of Project 1: (1) Childhood Asthma Management Program (CAMP) ancillary study; (2) Denver Asthma Panel Study (DAPS); and (3) Housing and Urban Development (HUD) ancillary study. Based on our CEHC’s research experience, progress and findings so far, we sought to strengthen the accuracy and relevance of our exposure assessments in DAPS by adding personal wearable exposure monitoring and bedroom air stationary monitoring. After EPA approval in January 2014, we successfully added and completed our Endotoxin Personal Exposure Monitoring Study (EPEM) to enhance, operationalize and validate our personal exposure monitoring for the longitudinal DAPS. Until recently, the lab assay for endotoxin has utilized limulus amebocyte lysate, a reagent that cross-reacts with molds. Now, there is an endotoxin assay based on recombinant Factor C, which only binds endotoxin. To advance scientific understanding of pure endotoxin exposure (i.e., independent of mold) and asthma outcomes, we developed and validated a Standard Operating Procedure to measure endotoxin using the rFC assay and established quality control parameters.
(1) Our CAMP investigation is the first study, to our knowledge, to clearly distinguish household endotoxin from mold exposure in children with asthma. By using the endotoxin-specific rFC assay and separate mold exposure measures (i.e., mold plate counts), we evaluated the effects of household endotoxin and mold exposures on asthma severity. In fact, we found that higher endotoxin levels in baseline dust samples (n = 962) were associated with fewer prednisone days (an indicator of severe asthma exacerbations), while high mold correlations were found between log mold concentrations and endotoxin levels (unadjusted r = 0.17; p = < 0.0001). When the effects of endotoxin and mold exposures on prednisone days were assessed for each site (with both exposures in the model), endotoxin-associated reductions and mold-associated increases in prednisone days were found for 5 of the 8 CAMP sites (Baltimore, Denver, San Diego, St. Louis, and Toronto), consistent with the overall model.
We also investigated the interaction of endotoxin with 48 SNPs in 11 Toll-Like Receptor (TLR) genes (TLR-1, -2, -3, -4, -5, -6, -9, -10, CD14, MyD88, LY96, ACAA1) on severe asthma exacerbations (i.e., at least one ER visit or hospitalization for asthma in the past year). This preliminary analysis was restricted to CAMP participants of Caucasian ethnicity: 517 CAMP Caucasian participants included 84 cases with at least one severe asthma exacerbation vs. 433 controls. For two SNPs (in TLR9 and MyD88), the presence of the dominant genotype and higher endotoxin levels increase the risk of severe asthma exacerbations. Because of these significant TLR gene-endotoxin interactions, we expanded our genotyping of genes downstream of the TLR receptor complex. We also performed a genome wide, pathway level analysis to develop a gene-by-environment model for endotoxin exposure and asthma exacerbations. Glycosphingolipid metabolism showed the most evidence for interaction with endotoxin exposure at the pathway level (FDR < 0.04) in models of asthma severity. Gene polymorphisms that contributed to glycosphingolipid pathway enrichment included interaction of endotoxin with polymorphisms in SPTLC2, ASAH1, GALC, ARSB, PPAP2B and SPTLC1. Other pathways and functional groupings that showed possible interactions with environmental endotoxin were monoamine G protein coupled receptors (including muscarinic receptors (CHRM3), histamine receptors (HRH1)) (FDR = 0.06), the nitric oxide synthase pathway (FDR = 0.08), and Fc Epsilon Receptor 1 signaling in mast cells (FDR=0.15). Pathway level analysis identified functional groupings of genes that may interact with ambient endotoxin exposure to alter asthma severity in children. These gene-by-environment interactions would not have been detected in a conventional genome wide survey of individual SNP-level gene-by-environment associations.
(2) For DAPS, we strengthened the accuracy and relevance of our exposure assessments, by adding personal wearable exposure monitoring and bedroom air stationary monitoring. With EPA approval in January 2014, we successfully added and completed the Endotoxin Personal Exposure Monitoring study (EPEM) to develop, enhance and operationalize personal exposure monitoring methods for DAPS. The DAPS protocol was developed to include these exposure monitoring enhancements. We received IRB approval and participant enrollment began in July 2014. Currently, 15 study participants have been enrolled, and 13 have completed the study (87% retention). Preliminary analyses were performed to validate exposure monitoring methods using the first study visit data from these early DAPS enrollees and additional EPEM study participants with asthma meeting DAPS criteria (n = 25). Clinical measures of asthma severity (spirometry, exhaled nitric oxide, and Composite Asthma Severity Index (CASI)) were assessed concurrent with deployment of monitors.
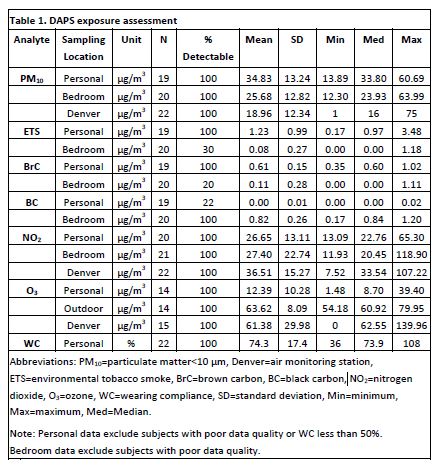
Exposure Samplers: The wearable Micro-miniature Personal Exposure Monitor (MicroPEM™, RTI International) and stationary Personal Environmental Monitor (PEM™, MSP Corp) samplers were used to measure particulate matter < 10 μm (PM10) via gravimetric analysis and real-time nephelometry as well as components of PM10, including black carbon (BC), brown carbon (BrC), and environmental tobacco smoke (ETS), via spectrophotometry. Ogawa™ passive badges (Ogawa USA) were used to measure O3 and NO2. Publically available Air Quality Index (AQI) data, from a Colorado Department of Public Health (CDPHE) air monitoring station in central Denver, were used for outdoor (ambient) comparisons of PM10, NO2, and O3. Table 1 presents summary statistics for exposure assessments.
Exposure Monitor Deployment and Clinical Asthma Severity Assessments: Ogawa™ passive badges and PEM™ monitors were installed in participants’ bedrooms. Ogawa™ passive badges also were installed outside of the home during the summer visit. Each participant was fitted with a personal monitoring apparatus (MicroPEM™, Ogawa™ passive badges, and iTrack Micro™ GPS device). All devices were retrieved after approximately 72 hours. Spirometry, exhaled nitric oxide (eNO), and Composite Asthma Severity Index (CASI) were assessed concurrent with deployment of monitors and badges (Wildfire, et al. J Allergy Clin Immunol. 2012; PubMed PMID: 22244599].
Feasibility of Exposure Assessment Using Wearable Monitors in Children: Wearing compliance (WC) was calculated from MicroPEM™ accelerometry data and the personal exposure log. Statistical analyses included participants with greater than 50% (“acceptable”) wearing compliance based on thresholds used in prior studies (Lawless P, Thornburg J, et al. J Expo Sci Environ Epidemiol. 2012; PubMed PMID: 22377684). WC was acceptable in 91% of participants, with a median WC of 78% for those with acceptable WC.
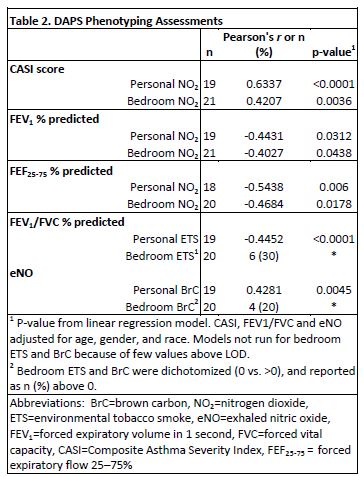
Environmental Exposures are Detectable and Correlate with Clinical Assessments: Personal versus stationary exposures were analyzed with linear mixed models or Wilcoxon signed rank tests depending on the statistical distributions. Personal exposure levels were significantly higher than stationary bedroom levels for PM10 (p = 0.025), BrC (p = 0.0005), and ETS (p < 0.0001). In contrast, personal levels for BC were significantly lower than stationary bedroom levels (p < 0.0001). Linear regression models assessed how pollutant levels were associated with asthma severity, and were adjusted for age, race and gender, when applicable. Linear regression analyses revealed multiple significant exposure-asthma severity correlations (Table 2). Personal exposure-asthma severity correlations were stronger than stationary exposure-asthma severity correlations, suggesting that personal monitors provide more accurate measurements of environmental exposures.
Personal Monitoring with the MicroPEM Has Been Validated as Better than Stationary Monitoring for Some Exposures. Preliminary DAPS analyses have shown that personal monitors measure NO2 more accurately, and provide better detection of ETS and BrC. As a result, the personal monitors reveal correlations between asthma severity and air pollutant exposures that are missed by conventional stationary monitors. Similarly, in a prior collaboration with RTI, research with backpack personal monitors demonstrated better endotoxin-asthma severity correlations than did stationary monitors. Greater exposure to personal endotoxin (1 EU/m3 change) was associated with more asthma symptoms during sleep (p = 0.04) and lower FEV1 (p = 0.04; correlation p = 0.15), but exposure to indoor stationary endotoxin was not significant (Rabinovitch N, Liu AH, et al. J Allergy Clin Immunol. 2005; PubMed PMID: 16275375). Personal monitoring has not yet been validated for allergens, glucans (molds), black carbon, or O3 with respect to exposure-asthma severity correlations. Therefore, we will continue to use standard methods, stationary air filters and dust sample collection, in addition to personal monitoring.
Collaborations Have Been Established. DAPS sub-studies have helped to develop and strengthen collaborative relationships for DAPS by piloting some of the proposed research with the investigators and exposure monitoring group involved in this study: Andrew Liu (Project 1 Leader and DAPS research team), Jonathan Thornburg (RTI International), and Anna Faino (Biostatistician, NJH).
These preliminary analyses have shown that personal monitors measure respirable pollutant exposures more accurately. As a result, the personal monitors reveal correlations between asthma severity and air pollutant exposures that are missed by conventional stationary monitors. These enhanced exposure monitoring methods have been incorporated into the DAPS study. These findings were selected for presentation at the 2015 American Academy of Allergy, Asthma & Immunology Annual Meeting (Best of Environmental and Occupational Respiratory Diseases Interest Section) and the 2015 American Thoracic Society International Conference, and are being developed for publication.
(3) For the HUD study, 115 participants were enrolled, and the study participant follow-up visits were completed in March 2012. The HUD cohort comprised mostly ethnic minority children, with asthma, living in low-income housing. The homes had many asthma triggers that were targets for remediation at three levels: Education Only, Minor Remediation, and Moderate Remediation. Endotoxin concentration was measured in 262 house dust samples collected from the general living area and participant’s bedroom. Seventy homes had all dust samples collected, meaning adequate dust samples for endotoxin measurement from both general living area and bedroom, before (Pre) and 6 months after (Post) remediation.
Project 2–ENDOTOXIN DETERMINANTS OF EARLY HOST RESPONSE TO RSV
This project has made substantial progress in achieving its overall objective to characterize the influence of postnatal ozone exposure on TLR expression and on airway structure and function. To the best of our knowledge, this is the first study to profile the transcriptome response of the newborn lung to acute ozone exposure using genome wide gene expression microarray analysis. The results identified several novel genes and molecular pathways never before associated with ozone exposure. The pattern of gene expression and the molecular pathways perturbed by ozone in the newborn lung are different from those described previously for adult (fully developed) lungs. Specifically, it was mainly cell cycle-associated functions including cell division/proliferation that were altered most significantly after acute ozone exposure in the developing newborn lung. It is not clear whether this suppression of the cell cycle/proliferation can lead to permanent damage to the lung (altered lung growth or altered structure and function) or if it is transient only, to allow repair of potentially damaged DNA from ozone exposure.
We also found that the airway response to ozone is age-dependent and this response is suppressed in the neonatal lung due to TLR-4 deficiency in this early age. The results also indicate that the neutrophilic airway response to ozone is dependent on TLR-4 signaling, whereas lung permeability is regulated by a mechanism independent of TLR-4 signaling. The role of neutrophils in ozone-mediated injury, whether pathogenic or protective, remains to be defined as planned in our future studies.
We also have focused on the effect of ozone on airway responsiveness to allergen exposure and RSV infection. Studies of the effects on allergen exposure showed that acute postnatal ozone exposure increased AHR but not airway inflammation or antibody response to subsequent exposure to house dust mite. We are repeating these studies to confirm the findings and to determine if the increased AHR is mediated through a neurogenic mechanism (e.g., via substance P-NK1 receptor pathway). Studies of the effects of postnatal ozone exposure on response to RSV infection are in progress and will determine if ozone alters innate or adaptive immunity to viral infection. Ozone is known to alter the defense mechanisms of the lung against bacterial infection, but its effects on respiratory viral infection are largely unknown. Therefore, we expect to obtain new information that will improve our understanding of the host response to viral infection during exposure to common air pollutants.
Project 3–ENDOTOXIN DETERMINANTS OF EARLY HOST DEFENSE
We completed characterization of the effect of in vivo ozone exposure on lung innate immune response to Pam3CYS, a TLR2/TLR6 agonist. Ozone pre-exposure resulted in (1) increased whole lung lavage (WLL) cell influx; (2) increased IL-6 and KC, and decreased MIP-1α and TNF-α; and (3) increased cell surface expression of TLR4, TLR2 and TLR1 on macrophages as a result of ozone alone or in combination with Pam3CYS. In addition, we demonstrated that ozone followed by Pam3CYS resulted in a large increase in phosphorylation of both p44/42 (Erk1/2) MAPK and JNK kinases and significant reduction in non-phosphorylated p44/42 MAPK at 4 hours post Pam3CYS. This enhanced signal associated with ozone/Pam3CYS treatment was not present at 24 hours. To further characterize the priming effect of ozone on innate immunity at the molecular level in an unbiased manner, we performed gene expression profiling on lung tissue of mice from the four exposure groups. Ozone exposure has the strongest effect on gene expression at the 4-hour time point with the effect diminishing by the 24-hour time point. Ozone pre-exposure prior to Pam3CYS treatment (O3/Pam3CYS vs. FA/Pam3CYS) enhanced induction of Trmt5 at the 4 hours and Cck at 24 hours. Expression of Ttk (4-hour time point), Pbp2, Gjb4, Ncapg, and Pbk (24-hour time point) also were increased in the O3/Pam3CYS vs. FA/Pam3CYS but significantly less than in the O3/saline vs. FA/saline comparison. The most significant result among downregulated genes is down-regulation at 24 hours of killer cell lectin-like receptors (Klra3, Klra8, Klra9, Klra10, Klra15, Klra21, Klra22, Klra23, Klrb1a, and Klrk1). Our study demonstrates that expression of TLRs on macrophage surface is a dynamic process that is influenced by ozone and that this process is associated with differential expression of a number of previously unexplored genes. This dynamic nature of TLR expression is likely more general and could be influenced by other components of air pollution and in cell types other than macrophages. This priming effect of air pollution and genes that are associated with the process also represent potential therapeutic targets for air pollutant exposure in the context of pulmonary infection or allergic airway inflammation.
To understand how epigenetic mechanisms alter dendritic cell function and contribute to the etiology of allergic airway disease, we are developing a line of investigation that examines epigenetic marks and transcriptional profiles in distinct lineages of DCs that exist within the lung and are recruited to the draining lymph nodes in response to allergic sensitization. Preliminary data demonstrate the approach we are taking to isolating these cells for epigenetic and gene expression studies.
To follow up on our published findings on the role of methyl donor diet in the development of allergic airway disease in mice, we compared allergic airway disease phenotypes between methylene-tetrahydrofolate reductase (MTHFR) deficient mice on a C57/Bl6 background to wild-type (WT) C57/Bl6 mice using a house dust mite (HDM) allergen model. In brief, mice received an intraperitoneal (i.p.) sensitization of 10 μg HDM or saline on days 0 and 7 followed by an intratracheal (i.t.) challenge of 5 μg HDM or saline on days 14 and 15. Forty-eight hours after the final challenge, total cells and eosinophils in the bronchoalveolar lavage were 5.76 fold and 7.87 lower, respectively, in HDM-treated MTHFR deficient compared to HDM-treated WT mice. Furthermore, HDM-treated MTHFR KO mice demonstrate a 1.64 fold (p<0.05) reduction in lung resistance compared to HDM-treated WT mice in response to inhaled methacholine. These results suggest that allergic airway disease may be suppressed through the loss of MTHFR.
Future Activities:
The coming year will include continuation of DAPS visits, analyses and preparation of our findings for presentation and manuscript submissions. Findings from our DAPS‐EPEM preliminary analyses were selected for presentation at the 2015 American Academy of Allergy, Asthma & Immunology Annual Meeting (Best of Environmental and Occupational Respiratory Diseases Interest Section) and the 2015 American Thoracic Society International Conference, and are being developed for publication. For the NIH‐funded CAMP study, the Endotoxin Exposure Working Group completed its analyses, presented this work at the 2014 American Academy of Allergy, Asthma & Immunology Annual Meeting (Best of Environmental and Occupational Respiratory Diseases Interest Section), and is in the final stages of manuscript preparation for submission of their findings. We completed our collaborative investigation of endotoxin exposure and asthma outcomes in 150 inner-city children in Baltimore (NIH-funded MAACS Study: PI E. Matsui) and a manuscript of our findings was published. We also completed a collaborative investigation of endotoxin exposure and early childhood wheezing phenotypes in a Denver inner city pre-school cohort (NIH-funded CAPS Study). This was presented in 2014 at a premier meeting, and it is in the final stages of manuscript preparation for submission. What we have learned from our CAMP, EPEM, MAACS, HUD and CAPS studies, and the other Projects and COTC in our CEHC, has informed and strengthened DAPS.
References:
Wildfire JJ, et al. Development and validation of the composite Asthma Severity Index—an outcome measure for use in children and adolescents. Journal of Allergy and Clinical Immunology 2012;129(3):694-701.
Lawless P, Thornburg J, Rodes C, Williams R. Personal exposure monitoring wearing protocol compliance: an initial assessment of quantitative measurement. Journal of Exposure Science and Environmental Epidemiology 2012;22(3):274-280.
Rabinovitch N, Liu AH, Zhang L, Rodes CE, Foarde K, Dutton SJ, Murphy JR, Gelfand EW. Importance of the personal endotoxin cloud in school-age children with asthma. Journal of Allergy and Clinical Immunology 2005;116(5):1053-1057.
Journal Articles: 31 Displayed | Download in RIS Format
| Other center views: | All 51 publications | 30 publications in selected types | All 30 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Alper S, Warg LA, De Arras L, Flatley BR, Davidson EJ, Adams J, Smith K, Wohlford-Lenane CL, McCray Jr PB, Pedersen BS, Schwartz DA, Yang IV. Novel Innate Immune Genes Regulating the Macrophage Response to Gram Positive Bacteria. Genetics 2016;204(1):327-336. |
R834515 (Final) |
Exit Exit |
|
|
Breton CV, Marsit CJ, Faustman E, Nadeau K, Goodrich JM, Dolinoy DC, Herbstman J, Holland N, LaSalle JM, Schmidt R, Yousefi P, Perera F, Joubert BR, Wiemels J, Taylor M, Yang IV, Chen R, Hew KM, Freeland DM, Miller R, Murphy SK. Small-magnitude effect sizes in epigenetic end points are important in children's environmental health studies:the Children's Environmental Health and Disease Prevention Research Center's Epigenetics Working Group. Environmental Health Perspectives 2017;125(4):511-526. |
R834515 (Final) R835436 (2017) R836159 (2018) |
|
|
|
Das R, Subrahmanyan L, Yang IV, van Duin D, Levy R, Piecychna M, Leng L, Montgomery RR, Shaw A, Schwartz DA, Bucala R. Functional polymorphisms in the gene encoding macrophage migration inhibitory factor are associated with gram-negative bacteremia in older adults. Journal of Infectious Diseases 2014;209(5):764-768. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
De Arras L, Seng A, Lackford B, Keikhaee MR, Bowerman B, Freedman JH, Schwartz DA, Alper S. An evolutionarily conserved innate immunity protein interaction network. Journal of Biological Chemistry 2013;288(3):1967-1978. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Gabehart K, Correll KA, Yang J, Collins ML, Loader JE, Leach S, White CW, Dakhama A. Transcriptome profiling of the newborn mouse lung response to acute ozone exposure. Toxicological Sciences 2014;138(1):175-190. |
R834515 (2011) R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C002 (2015) R834515C003 (2014) |
Exit Exit Exit |
|
|
Gabehart K, Correll KA, Loader JE, White CW, Dakhama A. The lung response to ozone is determined by age and is partially dependent on toll-like receptor 4. Respiratory Research 2015;16:117. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C002 (2015) R834515C003 (2014) |
Exit Exit Exit |
|
|
Gao Z, Dosman JA, Rennie DC, Schwartz DA, Yang IV, Beach J, Senthilselvan A. NOS3 polymorphism, lung function, and exposure in swine operations: results of 2 studies. Journal of Allergy and Clinical Immunology 2014;134(2):485-488. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Henao-Martinez AF, Agler AH, LaFlamme D, Schwartz DA, Yang IV. Polymorphisms in the SUFU gene are associated with organ injury protection and sepsis severity in patients with Enterobacteriacea bacteremia. Infection, Genetics and Evolution 2013;16:386-391. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit |
|
|
Jing J, Yang IV, Hui L, Patel JA, Evans CM, Prikeris R, Kobzik L, O'Connor BP, Schwartz DA. Role of macrophage receptor with collagenous structure in innate immune tolerance. Journal of Immunology 2013;190(12):6360-6367. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Kelada SN, Wilson MS, Tavarez U, Kubalanza K, Borate B, Whitehead GS, Maruoka S, Roy MG, Olive M, Carpenter DE, Brass DM, Wynn TA, Cook DN, Evans CM, Schwartz DA, Collins FS. Strain-dependent genomic factors affect allergen-induced airway hyperresponsiveness in mice. American Journal of Respiratory Cell and Molecular Biology 2011;45(4):817-824. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit |
|
|
Lai PS, Hofmann O, Baron RM, Cernadas M, Meng QR, Bresler HS, Brass DM, Yang IV, Schwartz DA, Christiani DC, Hide W. Integrating murine gene expression studies to understand obstructive lung disease due to chronic inhaled endotoxin. PLoS One 2013;8(5):e62910. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Long H, O'Connor BP, Zemans RL, Zhou X, Yang IV, Schwartz DA. The Toll-like receptor 4 polymorphism Asp299Gly but not Thr399Ile influences TLR4 signaling and function. PLoS One 2014;9(4):e93550 (10 pp.). |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Matsui EC, Hansel NN, Aloe C, Schiltz AM, Peng RD, Rabinovitch N, Ong MJ, Williams DL, Breysse PN, Diette GB, Liu AH. Indoor pollutant exposures modify the effect of airborne endotoxin on asthma in urban children. American Journal of Respiratory and Critical Care Medicine 2013;188(10):1210-1215. |
R834515 (2012) R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C001 (2015) R834515C002 (2014) R834515C003 (2014) R834510 (2014) |
Exit Exit Exit |
|
|
Oakes JL, O'Connor BP, Warg LA, Burton R, Hock A, Loader J, LaFlamme D, Jing J, Hui L, Schwartz DA, Yang IV. Ozone enhances pulmonary innate immune response to a Toll-like receptor-2 agonist. American Journal of Respiratory Cell and Molecular Biology 2013;48(1):27-34. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Szefler SJ, Dakhama A. New insights into asthma pathogenesis and treatment. Current Opinion in Immunology 2011;23(6):801-807. |
R834515 (2011) R834515 (Final) |
Exit |
|
|
Szefler SJ. Advancing asthma care: the glass is only half full! Journal of Allergy and Clinical Immunology 2011;128(3):485-494. |
R834515 (2011) R834515 (Final) |
Exit Exit Exit |
|
|
Szefler SJ. Advances in pediatric asthma in 2011: moving forward. Journal of Allergy and Clinical Immunology 2012;129(1):60-68. |
R834515 (2011) R834515 (Final) |
Exit Exit Exit |
|
|
Warg LA, Oakes JL, Burton R, Neidermyer AJ, Rutledge HR, Groshong S, Schwartz DA, Yang IV. The role of the E2F1 transcription factor in the innate immune response to systemic LPS. American Journal of Physiology-Lung Cellular and Molecular Physiology 2012;303(5):L391-L400. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Warg LA, Oakes JL, Burton R, Neidermyer AJ, Rutledge HR, Groshong S, Schwartz DA, Yang IV. The role of the E2F1 transcription factor in the innate immune response to systemic LPS. American Journal of Physiology-Lung Cellular and Molecular Physiology 2012; 303(5):L391-L400.. |
R834515 (Final) |
Exit Exit |
|
|
Warg LA, Oakes JL, Burton R, Neidermyer AJ, Rutledge HR, Groshong S, Schwartz DA, Yang IV. The role of the E2F1 transcription factor in the innate immune response to systemic LPS. American Journal of Physiology-Lung Cellular and Molecular Physiology 2012;303(5):L391-L400. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Yang IV, Alper S, Lackford B, Rutledge H, Warg LA, Burch LH, Schwartz DA. Novel regulators of the systemic response to lipopolysaccharide. American Journal of Respiratory Cell and Molecular Biology 2011;45(2):393-402. |
R834515 (2013) R834515 (2014) R834515 (2015) R834515 (Final) R834515C001 (2014) R834515C002 (2014) R834515C003 (2014) R834515C003 (2016) |
Exit Exit Exit |
|
|
Yang IV, Tomfohr J, Singh J, Foss CM, Marshall HE, Que LG, McElvania-Tekippe E, Florence S, Sundy JS, Schwartz DA. The clinical and environmental determinants of airway transcriptional profiles in allergic asthma. American Journal of Respiratory and Critical Care Medicine 2012;185(6):620-627. |
R834515 (Final) |
Exit Exit Exit |
|
|
Julian CG, Yang IV, Browne VA, Vargas E, Rodriguez C, Pedersen BS, Moore LG, Schwartz DA. Inhibition of peroxisome proliferator-activated receptor gamma: a potential link between chronic maternal hypoxia and impaired fetal growth. FASEB Journal 2014;28(3):1268-1279. |
R834515 (Final) |
Exit |
|
|
Kelada SN, Carpenter DE, Aylor DL, Chines P, Rutledge H, Chesler EJ, Churchill GA, Pardo-Manuel de Villena F, Schwartz DA, Collins FS. Integrative genetic analysis of allergic inflammation in the murine lung. American Journal of Respiratory Cell and Molecular Biology 2014;51(3):436-445. |
R834515 (Final) |
Exit Exit |
|
|
Liang L, Willis-Owen SAG, Laprise C, Wong KCC, Davies GA, Hudson TJ, Binia A, Hopkin JM, Yang IV, Grundberg E, Busche S, Hudson M, Ronnblom L, Pastinen TM, Schwartz DA, Lathrop GM, Moffatt MF, Cookson W. An epigenome-wide association study of total serum immunoglobulin E concentration. Nature 2015;520(7549):670-674. |
R834515 (Final) |
Exit |
|
|
Eyring KR, Pedersen BS, Yang IV, Schwartz DA. In Utero Cigarette Smoke Affects Allergic Airway Disease But Does Not Alter the Lung Methylome. PloS one 2015;10(12):e0144087 (10 pp.). |
R834515 (Final) |
Exit Exit |
|
|
Julian CG, Pedersen BS, Salmon CS, Yang IV, Gonzales M, Vargas E, Moore LG, Schwartz DA. Unique DNA Methylation Patterns in Offspring of Hypertensive Pregnancy. Clinical and Translational Science 2015;8(6):740-745. |
R834515 (Final) |
Exit Exit |
|
|
Yang IV, Pedersen BS, Liu A, O’Connor GT, Teach SJ, Kattan M, Misiak RT, Gruchalla R, Steinbach SF, Szefler SJ, Gill MA, Calatroni A, David G, Hennessy CE, Davidson EJ, Zhang W, Gergen P, Togias A, Busse WW, Schwartz DA. DNA methylation and childhood asthma in the inner city. Journal of Allergy and Clinical Immunology 2015;136(1):69-80. |
R834515 (Final) |
Exit Exit Exit |
|
|
Lai PS, Liang L, Cibas ES, Liu AH, Gold DR, Baccarelli A, Phipatanakul W. Alternate methods of nasal epithelial cell sampling for airway genomic studies. Journal of Allergy and Clinical Immunology 2015;136(4):1120-1123.e4. |
R834515 (Final) |
Exit Exit |
|
|
Yang IV, Richards A, Davidson EJ, Stevens AD, Kolakowski CA, Martin RJ, Schwartz DA. The nasal methylome: a key to understanding allergic asthma. American Journal of Respiratory and Critical Care Medicine 2017;195(6):829-831. |
R834515 (Final) |
Exit Exit Exit |
|
|
Yang IV, Pedersen BS, Liu AH, O'Connor GT, Pillai D, Kattan M, Misiak RT, Gruchalla R, Szefler SJ, Khurana Hershey GK, Kercsmar C, Richards A, Stevens AD, Kolakowski CA, Makhija M, Sorkness CA, Krouse RZ, Visness C, Davidson EJ, Hennessy CE, Martin RJ, Togias A, Busse WW, Schwartz DA. The nasal methylome and childhood atopic asthma. Journal of Allergy and Clinical Immunology 2017;139(5):1478-1488. |
R834515 (Final) |
Exit Exit Exit |
Supplemental Keywords:
endotoxin, exposure, children, asthma, risk, health effects, susceptibility, sensitive populations, genetic predisposition, genetic polymorphism, indoor air, dose-response, ozone, remediation, human health, health, biology, health risk assessment, children's health, allergens/asthma, asthma indices, intervention, Health, RFA, Scientific Discipline, HUMAN HEALTH, Health Risk Assessment, Biology, Allergens/Asthma, Children's Health, Health Effects, sensitive populations, airway inflammation, asthma, asthma indices, asthma triggers, children, endotoxin, allergic responseRelevant Websites:
Research Programs & Departments | National Jewish Health Exit
Progress and Final Reports:
Original Abstract Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R834515C001 Endotoxin Exposure and Asthma in Children
R834515C002 Environmental Determinants of Early Host Response to RSV
R834515C003 Environmental Determinants of Host Defense
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2015 Progress Report
- 2013 Progress Report
- 2012 Progress Report
- 2011 Progress Report
- 2010 Progress Report
- Original Abstract
30 journal articles for this center