Grantee Research Project Results
2011 Progress Report: Integrated Design, Modeling, and Monitoring of Geologic Sequestration of Anthropogenic Carbon Dioxide to Safeguard Sources of Drinking Water
EPA Grant Number: R834386Title: Integrated Design, Modeling, and Monitoring of Geologic Sequestration of Anthropogenic Carbon Dioxide to Safeguard Sources of Drinking Water
Investigators: McPherson, Brian J. , Solomon, Douglas Kip , Deo, Milind D. , Goel, Ramesh
Current Investigators: McPherson, Brian J. , Deo, Milind D. , Solomon, Douglas Kip , Goel, Ramesh
Institution: University of Utah
EPA Project Officer: Aja, Hayley
Project Period: December 1, 2009 through November 30, 2012 (Extended to November 30, 2013)
Project Period Covered by this Report: March 15, 2010 through March 14,2011
Project Amount: $899,567
RFA: Integrated Design, Modeling, and Monitoring of Geologic Sequestration of Anthropogenic Carbon Dioxide to Safeguard Sources of Drinking Water (2009) RFA Text | Recipients Lists
Research Category: Drinking Water , Water
Objective:
The specific objectives of our study are to: (1) identify risks specific to underground sources of drinking water (USDWs) and develop associated Probability Density Functions (PDFs), (2) quantify risks to USDWs by pressure/brine/CO2 migration through seals, (3) quantify risks to USDWs by lateral migration of pressure/brine/CO2, and (4) determine conditions that minimize (or eliminate) the risks to USDWs.
Progress Summary:
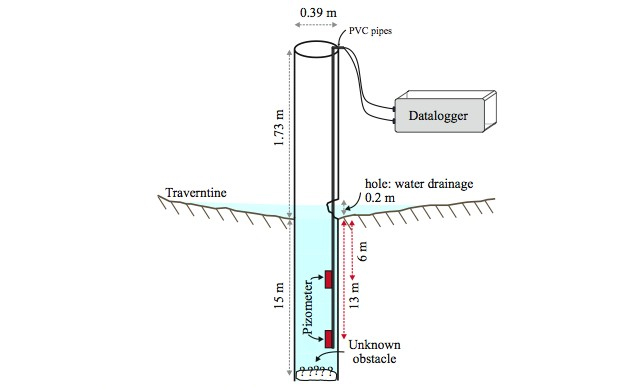
Figure 3. SChematic diagram of well configuraten and pizometer installation.
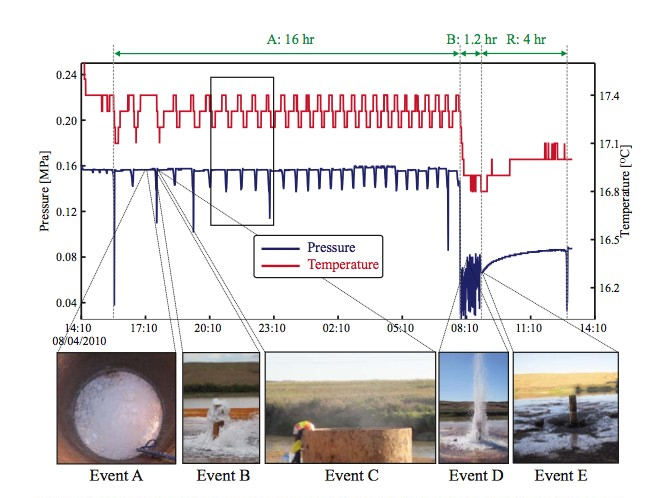
Figure 4. Time-series changes of pressure and temperature collected August 4-5 2010.
| Field Trip | Starting Time | Ending Time | Eruption Type | Duration | Comment |
|---|---|---|---|---|---|
| 8/4 - 8-6 | 8/4 - 15:30 pm | 8/5 - 7:30 am | A-type | 16 hours | |
| 8/5 - 7:30 am | 8/5 - 8:40 am | B-type | 1.2 hours | ||
| 8/5 - 8:40 am | 8/5 - 12:40 pm | Recharge | 4 hour | Because the levels of static pressure and temperature were changed, we stopped the recording at the pizometer. | |
| 8/5 - 12:40 pm | 8/5 - 18:15 pm | C-type | 5.6 hour | We began to record manually | |
| 8/5 - 18:15 pm | 8/6 - over 1:00 am (?) | Probably D-type | Over 4.8 hour (?) | Eruptions continued ov r1:00 am on 8/6. We do not know the exact time it stopped | |
| 8/6 - over 1:00 am (?) | 8/6 - 10:00 am | Recharge | At 10:00 am on August 6, the geyser was completely drained as shows in Event D of Figure 4 | ||
| 9/1 - 9/5 | 9/1 - 16:50 pm | 9/2 - 6:10 am | A-type | 13.3 hour | |
| 9/2 - 6:10 am | 9/2 - 7:10 am | B-type | 1 hour | ||
| 9/2 - 7:10 am | 9/2 - 10:00 am | Recharge | 2.8 hour | ||
| 9/2 - 10:00 am | 9/2 - 16:20 pm | C-type | 4.3 hour | ||
| 9/2 - 16:20 pm | 9/2 - 21:00 pm | D-type | 4.7 hour | PVC pipe was attached to pizometers are broken. The level of static pressure and temperature was changed slightly | |
| 9/2 - 21:00 pm | 9/3 - 7:10 am | Recharge | 10.2 hours | ||
| 9/3 - 7:10 am | 9/3 - 18:40 pm | A-type | 11.5 hour | ||
| 9/3 - 18:40 pm | 9/3 - 19:30 pm | B-type | 0.8 hour | ||
| 9/3 - 19:30 pm | 9/3 - 21:50 pm | Recharge | 2.3 hours | ||
| 9/3 - 21:50 pm | 9/4 - 3:50 am | C-type | 6 hours | ||
| 9/4 - 3:50 am | 9/4 - 9:10 am | D-type | 5.3 hours | ||
| 9/4 - 9:10 am | 9/4 - 19:50 pm | Recharge | 7.7 hours | ||
| 9/4 - 16:50 pm | 9/5 - 6:39 am | A-type | 10.7 hours | ||
| 9/5 - 6:30 am | 9/5 - 7:30 am | B-type | 1 hours |
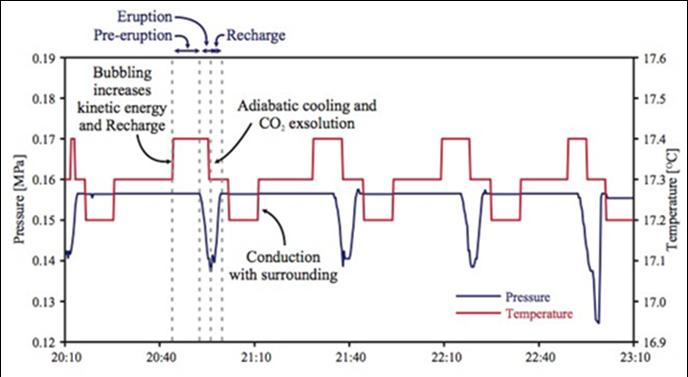
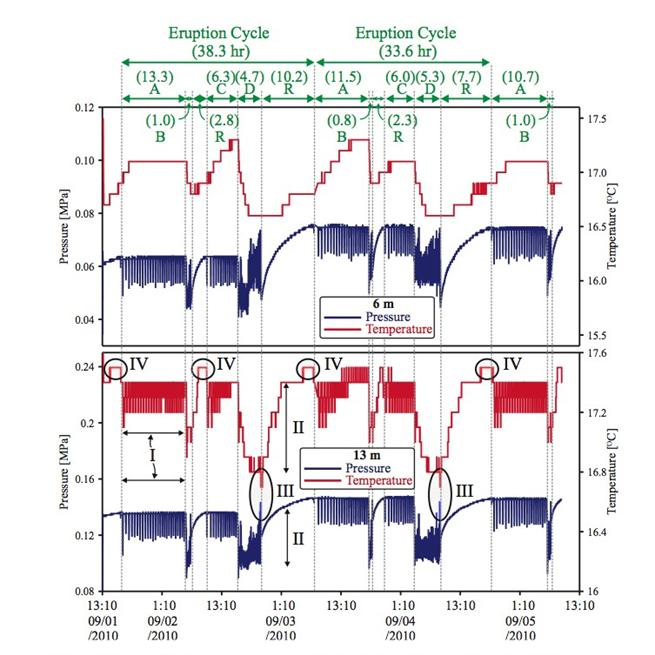
Figure 6. Time-series changes of pressure and temperature collected at September 1-5 2010
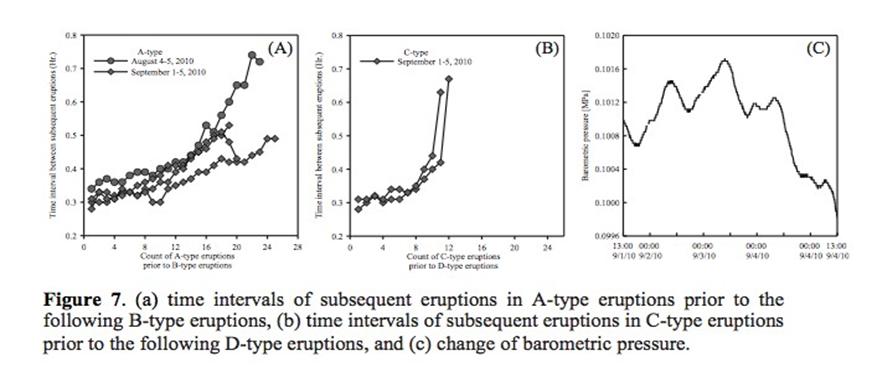
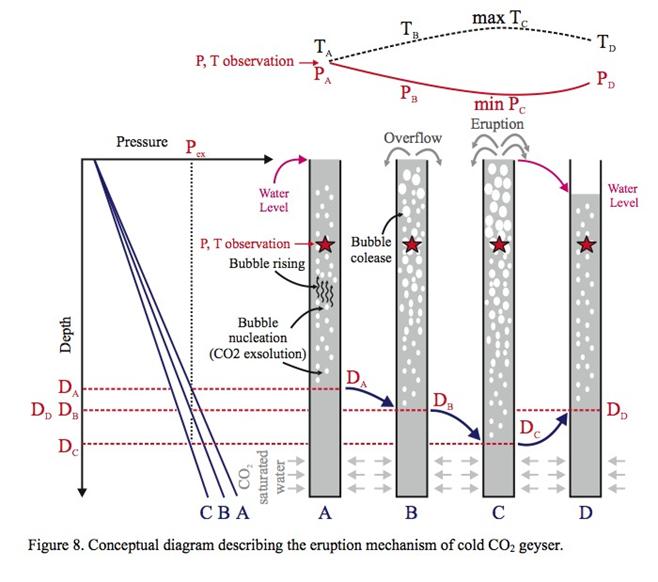
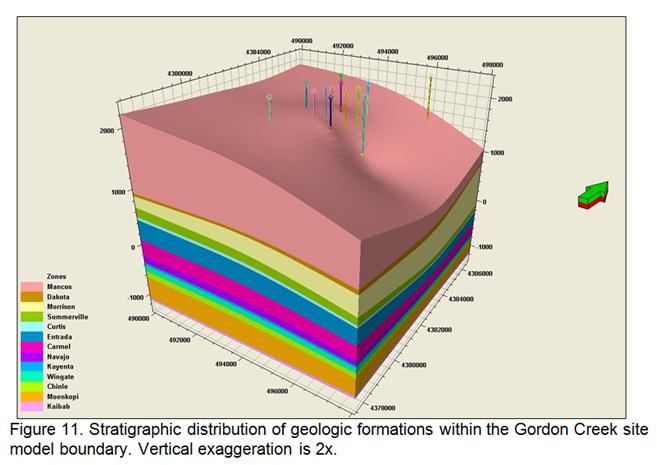
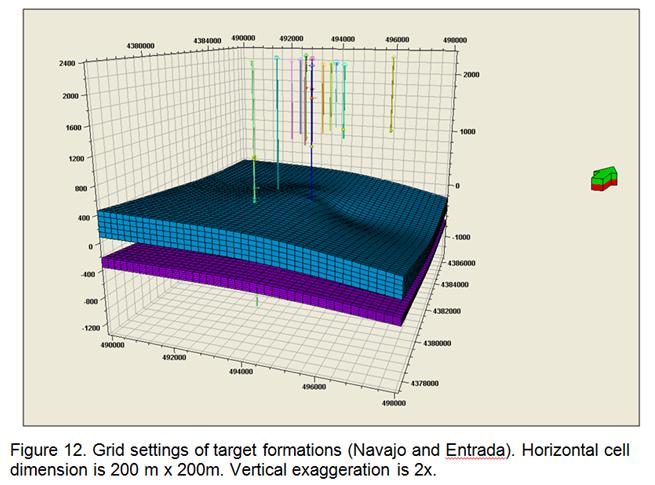
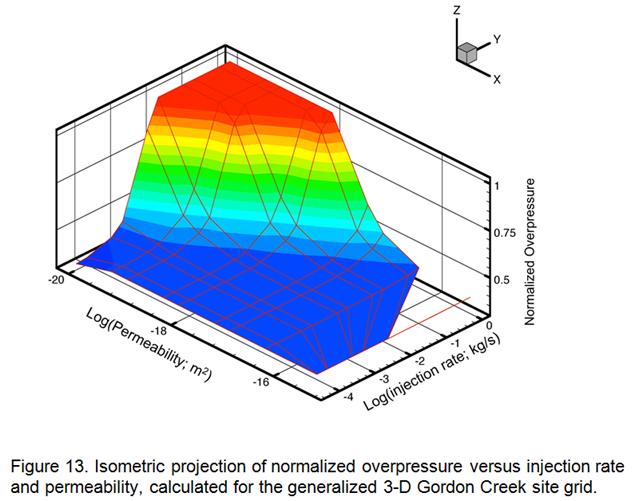
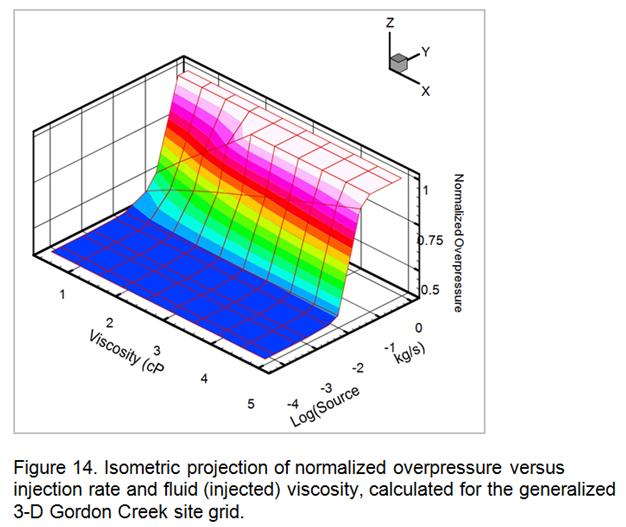
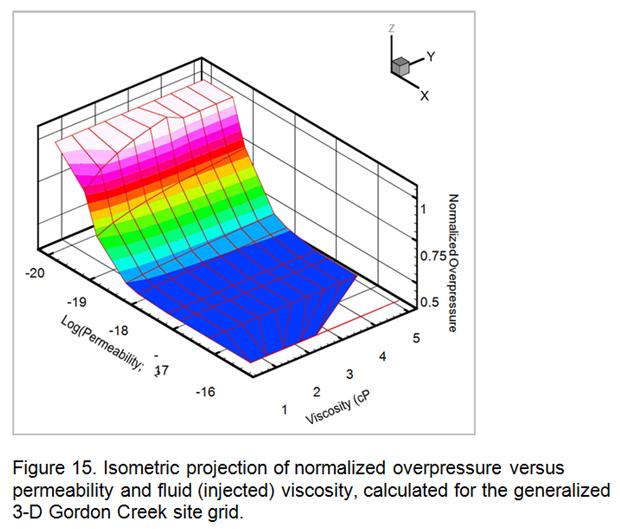
- Development of a methodology for calculating the probability of leakage of CO2 into USDWs, especially via known (or unknown) fault pathways; and
- A study of the impact of the fault structure on possible leakage of CO2 into overlying formations.
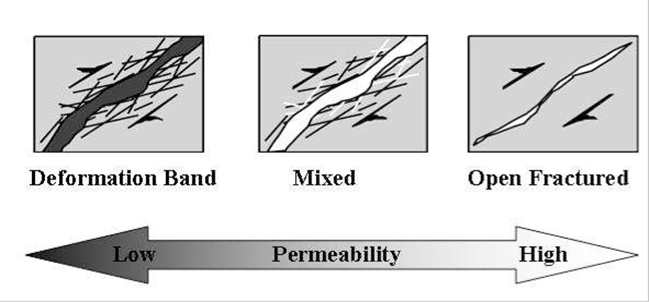
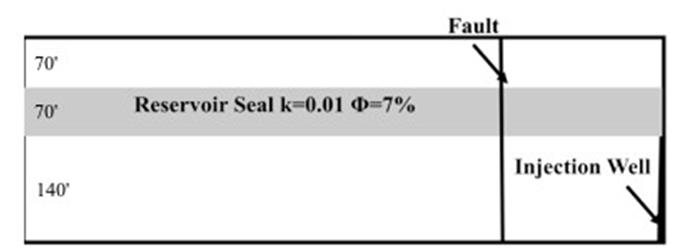
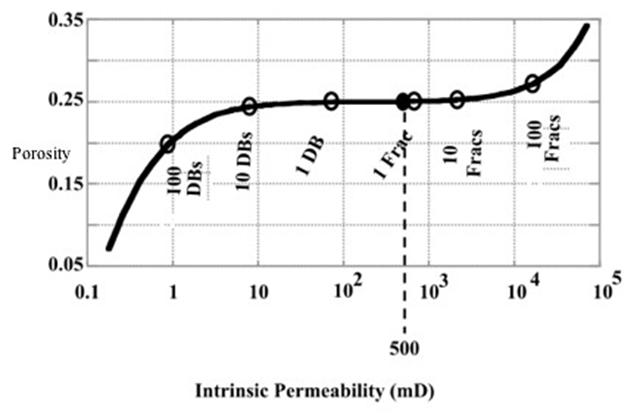
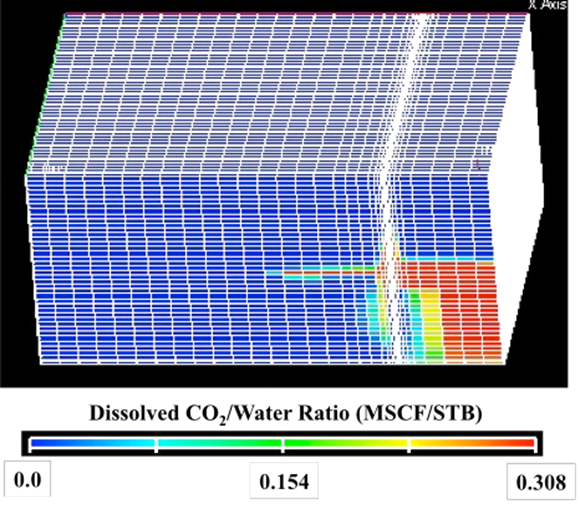
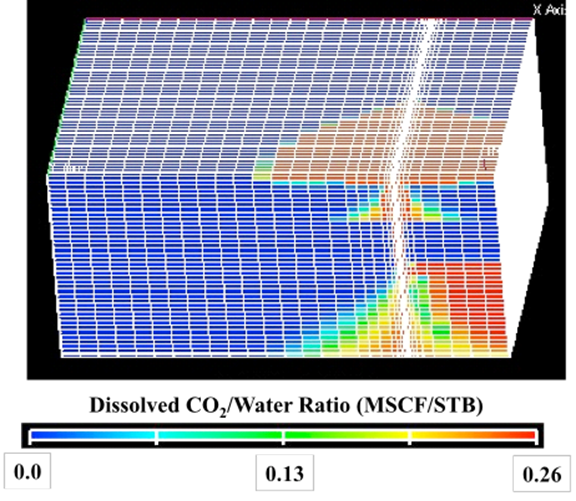
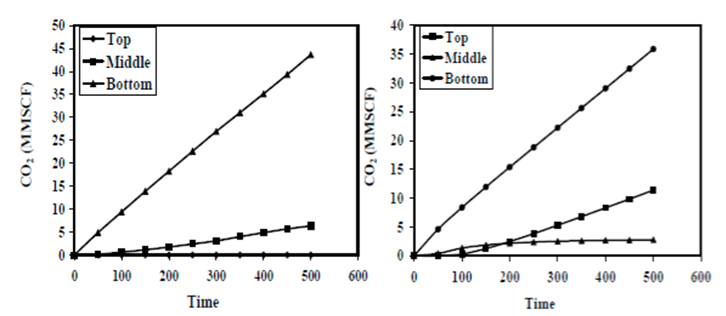

| Step | 4He (ccSTP/g) | 20Ne (ccSTP/g | R/Ra | Comments |
|---|---|---|---|---|
| Initial heating at 290 oC for 4.5 days | 1.59 X 10-7 | 2.45 X 10-8 | 0.108 | Total sample mass = 0.5390 g |
| Additional heating at 290 oC for 7 days | 1.41 X 10-8 | 1.99 X 10-8 | 0.148 | Result shows that approximately 90% of He was removed during initial heating |
| Filled to 3.596 Torr with pure helium, heated to 290 oC for 11 days. Placed in new container and heated at 290 oC for 23 days. | 8.76 X 10-7 | 1.36 X 10-8 | 0.169 | Mass placed in new container = 0.3068 g |
V1 is volume of helium (at STP) per cm3 fo quartz tested (determined by measuring mass and a standards density of 2.67 g/cc for quartz
p1 = standard pressure for gasses (1 atm)
p2 = helium pressure in the vessel
T1 = standard temperature fo rgases (273.15 K)
T2 = inpregnation temperature (563 K)
- Cw = S (Cqtz/V2) (Tfrm/T1) where,
Cqtz = concentration of helium in quartz (before the impregnation test)
V2 = helium accessible volume
Tfrm = temperature in the caprock formation at the location of the sample (estimated using the geothermal gradient at the site along with field measurements)
T1 = standard temperature for gases (273.15 K)
- Develop an interactive website for technology transfer and fundamental communication of results to the public (synergistic work with NSF project).
- Develop science curriculum materials for school districts in reference sites (synergistic work with NSF project).
- Develop press releases for local commercial media at reference sites (nothing reported this year).
- Develop print materials for broad distribution (nothing reported this year).
Future Activities:
References:
Journal Articles:
No journal articles submitted with this report: View all 3 publications for this projectProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
