Grantee Research Project Results
2009 Progress Report: Interactions of Natural Organic Matter with C60 Fullerene and their Impact on C60 Transport, Bioavailability and Toxicity
EPA Grant Number: R834093Title: Interactions of Natural Organic Matter with C60 Fullerene and their Impact on C60 Transport, Bioavailability and Toxicity
Investigators: Li, Qilin , Alvarez, Pedro J.
Institution: Rice University
EPA Project Officer: Hahn, Intaek
Project Period: January 1, 2009 through December 31, 2011
Project Period Covered by this Report: July 1, 2008 through June 30,2011
Project Amount: $399,995
RFA: Exploratory Research: Nanotechnology Research Grants Investigating Fate, Transport, Transformation, and Exposure of Engineered Nanomaterials: A Joint Research Solicitation - EPA, NSF, & DOE (2007) RFA Text | Recipients Lists
Research Category: Nanotechnology , Safer Chemicals
Objective:
The objectives of this research are to (1) understand the chemical nature of NOM C60 interactions and their impact on solubility and physicochemical properties of nC60 in realistic aqueous environments; (2) determine the effect of NOM on nC60 adsorption/deposition and transport in surface water and groundwater systems; and (3) investigate the effect of NOM-C60 interactions on nC60 toxicity mechanisms. During our investigation, solar irradiation was found to greatly alter the physicochemical properties of nC60, and consequently influence its fate, transport, and toxicity. Another type of carbon based nanomaterials, carbon nanotubes, were also found to undergo photochemical transformation under sunlight. As a result, we adjusted our focus to include the investigation on the role of sunlight and expanded our scope of research to include carbon nanotubes.
Progress Summary:
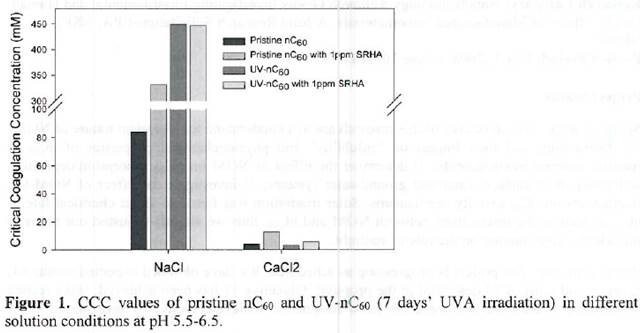
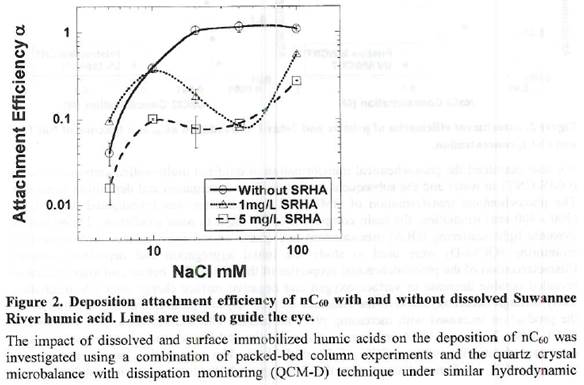
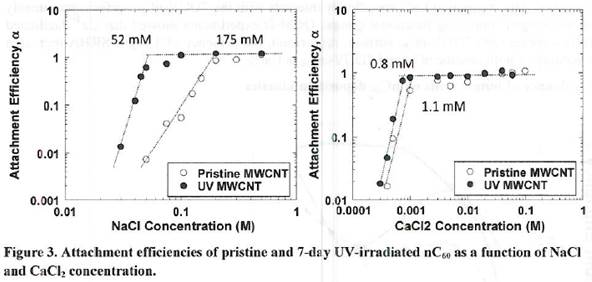
Future Activities:
We will continue to investigate the impact of NOM on the environmental fate and transport of nC60 nanoparticles. Research will focus on the role of humic acids on heterogeneous aggregation and deposition kinetics of fullerene nanoparticles. Natural river sediment samples have been collected from Buffalo Bayou, Houston, TX, and will be collected from two more locations where sediment properties differ. Heterogeneous aggregation of nC60 with river sediment will be investigated.
Journal Articles on this Report : 3 Displayed | Download in RIS Format
| Other project views: | All 13 publications | 5 publications in selected types | All 5 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Hwang YS, Qu X, Li Q. The role of photochemical transformations in the aggregation and deposition of carboxylated multiwall carbon nanotubes suspended in water. Carbon 2013;55:81-89. |
R834093 (2009) R834093 (2010) R834093 (Final) |
Exit |
|
|
Qu X, Hwang YS, Alvarez PJJ, Bouchard D, Li Q. UV irradiation and humic acid mediate aggregation of aqueous fullerene (nC60) nanoparticles. Environmental Science & Technology 2010;44(20):7821-7826. |
R834093 (2009) R834093 (Final) |
Exit Exit Exit |
|
|
Qu X, Alvarez PJJ, Li Q. Impact of sunlight and humic acid on the deposition kinetics of aqueous fullerene nanoparticles (nC60). Environmental Science & Technology 2012;46(24):13455-13462. |
R834093 (2009) R834093 (2010) R834093 (Final) |
Exit Exit Exit |
Supplemental Keywords:
Fullerene nanoparticles, nanomaterials, natural organic matter, UV, irradiation, transport, fate, bioavailability, toxicity, photochemical transformation, aggregation, deposition, sorption, stability, Health, Scientific Discipline, Water, Health Risk Assessment, Risk Assessments, Environmental Chemistry, Engineering, Chemistry, & Physics, Biochemistry, Drinking Water, community water system, toxicity, toxicokinetics, epidemelogy, nanomaterials, water quality, human exposure, engineered nanomaterials, drinking water system, drinking water contaminants, fate and transport, ambient particle health effects, nanotechnology, other - risk assessment, cellular responses, human health effects, human health risk, particle exposure, biochemical researchProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
