Grantee Research Project Results
2010 Progress Report: Production of Secondary Organic Aerosol from Multiphase Terpene Photooxidation
EPA Grant Number: R833750Title: Production of Secondary Organic Aerosol from Multiphase Terpene Photooxidation
Investigators: Shepson, Paul
Institution: Purdue University
EPA Project Officer: Chung, Serena
Project Period: November 1, 2007 through October 31, 2010 (Extended to October 31, 2011)
Project Period Covered by this Report: November 1, 2009 through October 31,2010
Project Amount: $333,397
RFA: Sources and Atmospheric Formation of Organic Particulate Matter (2007) RFA Text | Recipients Lists
Research Category: Particulate Matter , Air Quality and Air Toxics , Air
Objective:
The objective of this research is to improve our quantitative and mechanistic understanding of the production of secondary organic aerosol, an important type of air pollutant, from the atmospheric oxidation of α- and β-pinene. We aim to determine product yields for major gas phase OH- and O3-induced oxidation products with much smaller uncertainty bounds than have been reported previously, thereby improving the capability of air quality models that simulate aerosol production from BVOCs. We will study the oligomerization of aerosol phase species, and study the extent to which photochemistry in aerosols and in cloud water contributes to secondary organic aerosol production. The information produced from the linked laboratory and field studies will be used to develop improved computer model modules that describe secondary organic aerosol from these important terpenes.
Progress Summary:
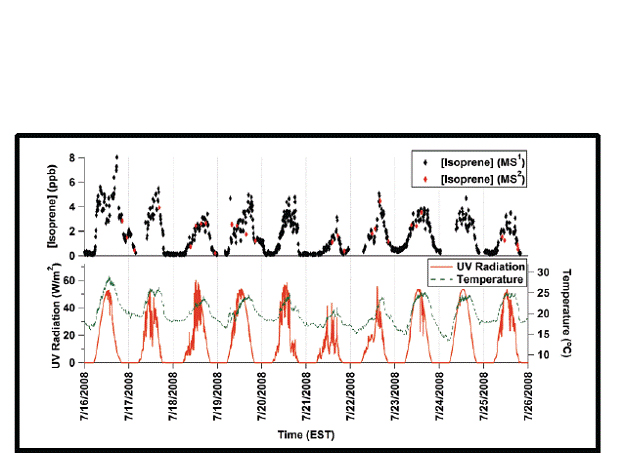
Figure 1. Comparison of PTRMS and PTRLIT modes of operation of PTRLIT, for isoprene at UMBS.
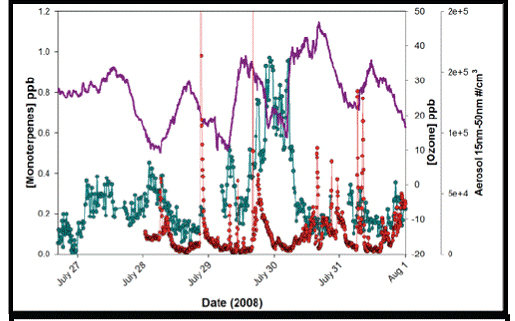
Figure 2. Aerosol formation event (red) in the presence of elevated monoterpenes (green)
and ozone (purple)
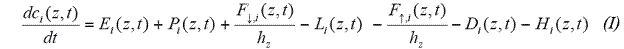
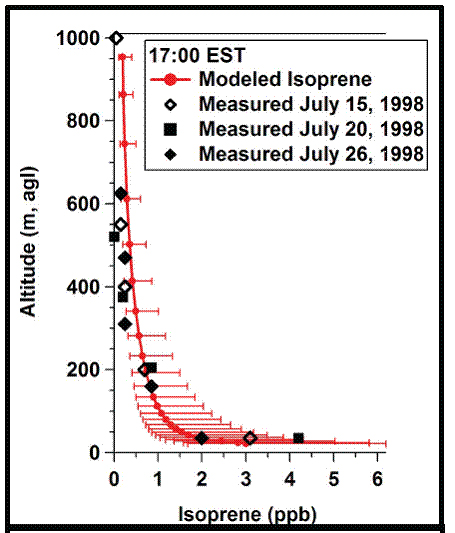
Figure 3. Comparison of 1D model isoprene with aircraft mesurement data.
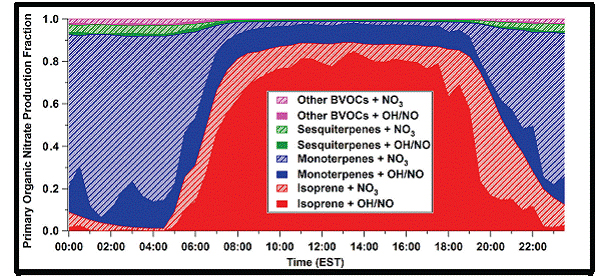
Figure 4. Fraction of the total organic nitrate production rate as a function of time of day.
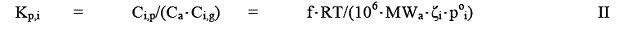
Figure 5. Terpene nitrate isomers.
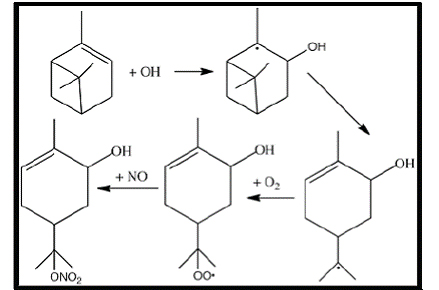
Figure 6. Mechanism for productoin of α-pinene hydroxy nitrate.

Figure 7. Cloud water collector on the Purdue Airborne
Laboratory for Atmospheric Research (ALAR)
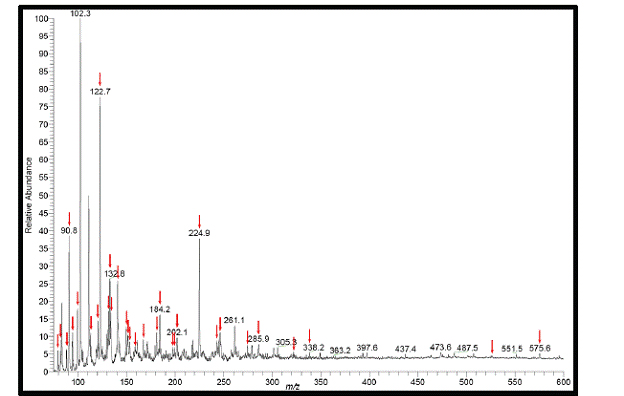
Figure 8. (+) ESI-MS spectrum of cloud water from 7,000 ft. Peaks labled with red arrows are absent
from the blank.
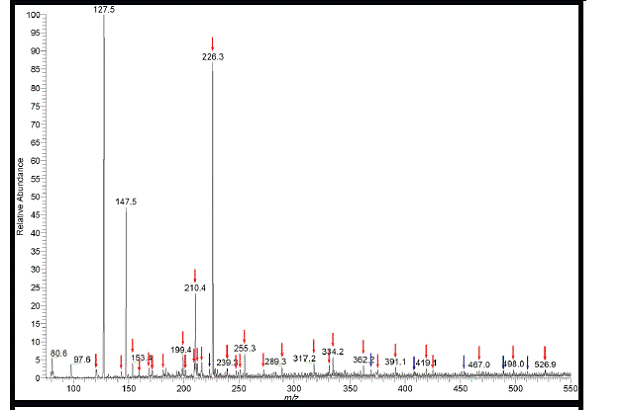
Figure 9. (-) ESI-MS spectrum of cloud water from 7,000 ft. Peaks labled with red arrows are absent
from the blank. Peaks labled with blue arrows are not in the blank but are present in the 80 Da neutral
loss spectruym which indicates that these species are organosulfates.
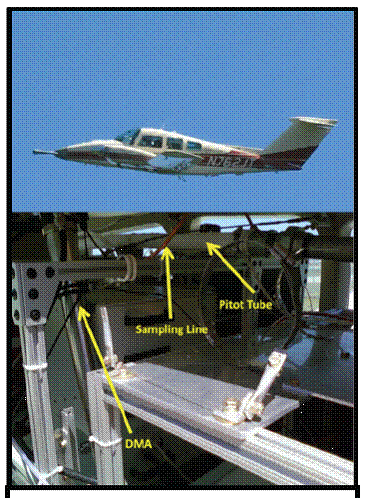
Figure 10. ALAR aircraft (top), with SMPS installed (bottom).
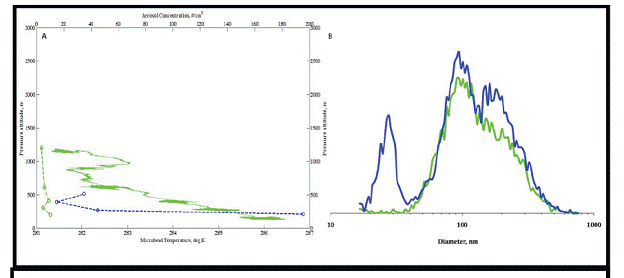
Figure 11. Vertical profile of temperature and aerosol number concentrations of Aitken mode particles (20-40nm)
over UMBS forest (green) and Lake Michigan (blue) for July 26 flight. Dashed lines represent vertical profile
of aeorsol. (B) Average aerosol size distribution spectrum for spectra recorded over land (green) and lake (blue).
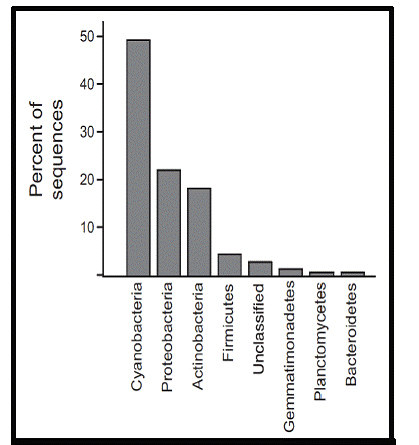
Figure 12. Classification of sequences from cloud water samples
acquired in 2008.
Future Activities:
- We are focusing on detection of specific lake water "markers" in the aerosol, for example, those from fresh water algae, e.g., palmitoleic acid. Graduate student Nick Veselka is completing a Master's degree on this problem.
- Smog chamber experiments. These experiments are focused on measurements of the yields and fates of organic nitrates from OH and NO3 reaction with α-pinene, as discussed above.
- 1D model development for SOA production from a suite of BVOCs. This will continue to be improved and developed.
Journal Articles on this Report : 6 Displayed | Download in RIS Format
| Other project views: | All 14 publications | 7 publications in selected types | All 7 journal articles |
|---|
| Type | Citation | ||
|---|---|---|---|
|
|
Alaghmand M, Shepson PB, Starn TK, Jobson BT, Wallace HW, Carroll MA, Bertman SB, Lamb B, Edburg SL, Zhou X, Apel E, Riemer D, Stevens P, Keutsch F. The morning NOx maximum in the forest atmosphere boundary layer. Atmospheric Chemistry and Physics Discussions 2011;11(10):29251-29282. |
R833750 (2010) R833750 (Final) |
Exit Exit |
|
|
Kourtev PS, Hill KA, Shepson PB, Konopka A. Atmospheric cloud water contains a diverse bacterial community. Atmospheric Environment 2011;45(30):5399-5405. |
R833750 (2010) R833750 (Final) |
Exit Exit Exit |
|
|
Mays KL, Shepson PB, Stirm BH, Karion A, Sweeney C, Gurney KR. Aircraft-based measurements of the carbon footprint of Indianapolis. Environmental Science & Technology 2009;43(20):7816-7823. |
R833750 (2009) R833750 (2010) R833750 (Final) |
Exit Exit Exit |
|
|
Mielke LH, Pratt KA, Shepson PB, McLuckey SA, Wisthaler A, Hansel A. Quantitative determination of biogenic volatile organic compounds in the atmosphere using proton-transfer reaction linear ion trap mass spectrometry. Analytical Chemistry 2010;82(19):7952-7957. |
R833750 (2009) R833750 (2010) R833750 (Final) |
Exit Exit Exit |
|
|
Muller M, Mielke LH, Breitenlechner M, McLuckey SA, Shepson PB, Wisthaler A, Hansel A. MS/MS studies for the selective detection of isomeric biogenic VOCs using a Townsend Discharge Triple Quadrupole Tandem MS and a PTR-Linear Ion Trap MS. Atmospheric Measurement Techniques 2009;2(2):703-712. |
R833750 (2009) R833750 (2010) R833750 (Final) |
Exit Exit |
|
|
Slade JH, VanReken TM, Mwaniki GR, Bertman S, Stirm B, Shepson PB. Aerosol production from the surface of the Great Lakes. Geophysical Research Letters 2010;37(18):L18807 (5 pp.). |
R833750 (2010) R833750 (Final) |
Exit Exit |
Progress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
