Grantee Research Project Results
2008 Progress Report: Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
EPA Grant Number: R832415C003Subproject: this is subproject number 003 , established and managed by the Center Director under grant R832415
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: Rochester PM Center
Center Director: Oberdörster, Günter
Title: Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
Investigators: Frampton, Mark W. , Zareba, Wojciech , Utell, Mark J. , Oakes, David , Phipps, Richard , Gelein, Robert
Current Investigators: Frampton, Mark W. , Utell, Mark J. , Zareba, Wojciech , Phipps, Richard , Gelein, Robert , Oakes, David
Institution: University of Rochester
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2012)
Project Period Covered by this Report: October 1, 2007 through September 30,2008
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
The overall objective of our current and planned studies is to determine the pulmonary and cardiovascular effects of exposure to ultrafine and fine particulate matter (PM). The clinical studies in healthy humans and susceptible individuals with diabetes proposed in this research core focus on the effects of ambient ultrafine and fine particles on three major determinants of adverse cardiac events: 1) blood coagulation induced by effects on platelets and circulating microparticles; 2) cardiac output; and 3) cardiac rhythm and repolarization.
Our overall hypothesis is that inhalation of ambient PM causes small but measurable changes in coagulation and cardiovascular function that help explain the cardiovascular effects of PM exposure. We further hypothesize that the cardiovascular effects are determined by the ability of PM to generate reactive oxygen and nitrogen species, and are more pronounced in subjects with type 2 diabetes. Inhaled ultrafine particles increase the burden of reactive oxygen species to the endothelium. Endothelial activation and vasoconstriction increase platelet adherence and release of thromboxane, activate and prolong the transit time of blood leukocytes, and deplete vascular nitric oxide (NO). Particles may also have direct effects on platelets and leukocytes. Vascular injury triggers release of procoagulant microparticles into the blood, and initiation of coagulation. In collaboration with the Vascular and Inflammation Facility Core, we measure the effects of inhaled ambient fine PM on platelet number, phenotype, and function, and quantitate intravascular microparticles derived from platelets and endothelial cells. In collaboration with the Cardiac Core, we use noninvasive monitoring methods to measure exposure effects on cardiac output, rhythm, and repolarization. These studies take advantage of and extend upon our project funded by the National Institute of Environmental Health Sciences and the EPA, “Ultrafine Particle-Induced Oxidative Stress”, which focuses on effects on vascular function and NO.
In Rochester, New York 75 patients taking part in a cardiac rehabilitation program will be studied. Patients from an active cardiac rehabilitation program within the University of Rochester Medical Center are offered enrollment in the health effects study as they enter the Cardiac Rehabilitation program. These are patients who have had a recent coronary event such as myocardial infarction or unstable angina leading to coronary stenting. The program involves supervised, graded twice or thrice weekly exercise sessions for a total of 10 weeks. As part of the rehabilitation protocol, vital signs and a standard 12 lead EKG will be performed. In addition to the regularly electrophysiologically monitored exercise of the rehabilitation program, subjects will undergo continuously recorded Holter ECG recordings performed and analyzed by the Cardiac Core allowing evaluation of a series of ECG parameters at rest, during exercise, and during immediate post-exercise period. Venous blood samples will be obtained once per week and analyzed by the Vascular & Inflammation Core for acute phase reactants (fibrinogen and C-reactive protein) previously found to vary with ultrafine particle exposure, as well as complete blood counts. Concurrently, ultrafine particle number and particle mass will be measured continuously at a central measuring site in downtown Rochester. Other EPA Criteria Pollutants are also measured in eastern Rochester as well. In addition, one-third of the patients are being asked to do personal particle count monitoring in their car to and from the rehab facility and in their homes for 48 hours using a portable nuclei counter (TSI model 3781). Levels of ambient ultrafine and fine particles will then be associated with health data from the cardiac rehabilitation panel study.
Approach:
Three human exposure protocols will be conducted using the Harvard ultrafine ambient particle concentrator, in collaboration with the Aerosol Generation and Analysis Facility Core. The first protocol will examine effects in healthy subjects, the second protocol will examine effects in age-matched subjects with type 2 diabetes, and the third protocol will assess the role of pretreatment with the cyclooxygenase inhibitor aspirin in preventing the cardiovascular effects of ultrafine/fine particle exposure. In collaboration with the Vascular and Inflammation Facility Core, we will determine particle effects on platelet function and release of endothelial and platelet microparticles into the circulation, and will examine effects on platelet-leukocyte adhesion, bone marrow stimulation, and changes in gene expression in blood mononuclear cells. Continuous ECG monitoring, in collaboration with the Cardiac Facility Core, will detect changes in cardiac repolarization, and noninvasive impedence cardiography will measure changes in cardiac output. Genomic DNA from exposed subjects will be analyzed for candidate gene polymorphisms identified in Research Core #2, Epidemiological Studies. Exposure studies will be designed and conducted in parallel with similar animal exposure studies conducted by Research Core #4, Animal Models. The impact of PM-associated reactive oxygen species, size, composition, and source will be examined in collaboration with Research Core #1, Characterization and Source Apportionment, and with the Biostatistics Facility Core.
Progress Summary:
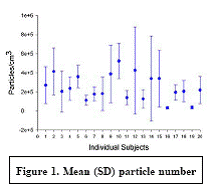
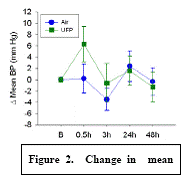
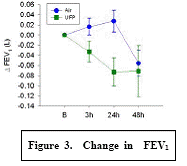
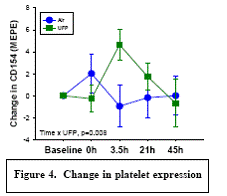
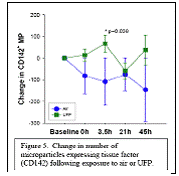
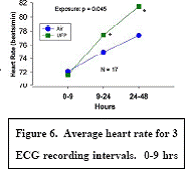
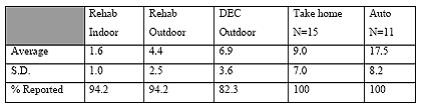
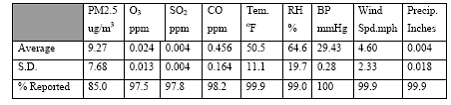
Expected Results:
Confirmation of our hypothesis that exposure to ambient ultrafine and fine particles promotes coagulation and alters cardiac function will have important implications for air pollution regulatory efforts, and will provide new approaches for the prevention of cardiovascular health effects.
Future Activities:
Journal Articles:
No journal articles submitted with this report: View all 57 publications for this subprojectSupplemental Keywords:
ultrafine particles; endothelial dysfunction; air pollution, cardiovascular health,, Health, RFA, Scientific Discipline, Air, PHYSICAL ASPECTS, Health Risk Assessment, Physical Processes, Risk Assessments, particulate matter, human exposure, long term exposure, aersol particles, atmospheric particles, ambient particle health effects, exposure, atmospheric aerosol particles, PM, ultrafine particulate matter, atmospheric particulate matter, acute cardiovascular effects, cardiovascular disease, human health riskProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832415 Rochester PM Center Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832415C001 Characterization and Source Apportionment
R832415C002 Epidemiological Studies on Extra Pulmonary Effects of Fresh and Aged Urban Aerosols from Different Sources
R832415C003 Human Clinical Studies of Concentrated Ambient Ultrafine and Fine Particles
R832415C004 Animal models: Cardiovascular Disease, CNS Injury and Ultrafine Particle Biokinetics
R832415C005 Ultrafine Particle Cell Interactions In Vitro: Molecular Mechanisms Leading To Altered Gene Expression in Relation to Particle Composition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2011 Progress Report
- 2010 Progress Report
- 2009 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
41 journal articles for this subproject
Main Center: R832415
191 publications for this center
144 journal articles for this center
