Grantee Research Project Results
2008 Progress Report: Project 1 -- Pulmonary Metabolic Response
EPA Grant Number: R832414C001Subproject: this is subproject number 001 , established and managed by the Center Director under grant R832414
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
Center: UC Davis Center for Children's Environmental Health and Disease Prevention
Center Director: Van de Water, Judith
Title: Project 1 -- Pulmonary Metabolic Response
Investigators: Winkle, Laura Van , Fanucchi, Michelle V. , Buckpitt, Alan
Current Investigators: Fanucchi, Michelle V. , Winkle, Laura Van , Buckpitt, Alan , Plopper, Charles
Institution: University of California - Davis
EPA Project Officer: Chung, Serena
Project Period: October 1, 2005 through September 30, 2010 (Extended to September 30, 2011)
Project Period Covered by this Report: October 1, 2007 through September 30,2008
RFA: Particulate Matter Research Centers (2004) RFA Text | Recipients Lists
Research Category: Human Health , Air
Objective:
Rationale:
We will apply transdisciplinary approaches to test these hypotheses, including histological, biochemical, and microarray analyses. Using defined synthetic particles consisting of graphitic carbon, a PAH, and a transitional metal, we will compare responses in intact airways at different sites of susceptibility (branch points and airway wall) in postnatal and adult rats to responses in human airway epithelial cell lines. As the Architecture Development Project defines the sites of deposition, we will adjust the sites that we are evaluating in the lung accordingly.
Progress Summary:

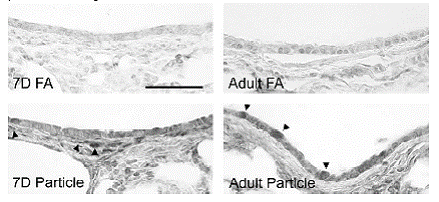
Expected Results:
Humans are exposed to multiple compounds early in life, yet most toxicological studies focus on the effects in adults. In addition, decisions regarding acceptable levels of environmental contaminants are based on adult data, which may not translate to children, the most susceptible portion of the population. Our work has already demonstrated that exposure to bioactivated pollutants produces much higher pulmonary toxicity in neonates than in adults. These studies will further define the role of particles in neonatal pulmonary susceptibility to environmental pollutants. Understanding the changes in lung cells following particle exposure at the gene and protein expression level will provide a basis for the development of biomarkers for assessing exposure and toxicity in young children.
Future Activities:
Journal Articles on this Report : 2 Displayed | Download in RIS Format
| Other subproject views: | All 43 publications | 10 publications in selected types | All 8 journal articles |
|---|---|---|---|
| Other center views: | All 128 publications | 71 publications in selected types | All 64 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Day KC, Plopper CG, Fanucchi MV. Age-specific pulmonary cytochrome P-450 3A1 expression in postnatal and adult rats. American Journal of Physiology-Lung Cellular and Molecular Physiology 2006;291(1):L75-L83. |
R832414 (2009) R832414C001 (2007) R832414C001 (2008) R832414C001 (Final) |
Exit Exit Exit |
|
|
Kennedy IM. The health effects of combustion-generated aerosols. Proceedings of the Combustion Institute 2007;31(2):2757-2770. |
R832414 (2009) R832414C001 (2008) R832414C001 (Final) |
Exit |
Supplemental Keywords:
ambient air, health effects, vulnerability, susceptibility, sensitive population, ozone, exposure, human health, metabolism, sensitive populations, infants, children, metals, oxidants, agriculture, transportation,, Health, RFA, Scientific Discipline, Air, Health Risk Assessment, Risk Assessments, particulate matter, Environmental Chemistry, Epidemiology, transport and fate, ambient aerosol, lung disease, endothelial function, human exposure, airborne particulate matter, long term exposure, pariculate matter, ambient particle health effects, epidemiological studies, PM, ultrafine particulate matter, toxicology, human health risk, lung injury, particle exposure, airway diseaseProgress and Final Reports:
Original AbstractMain Center Abstract and Reports:
R832414 UC Davis Center for Children's Environmental Health and Disease Prevention Subprojects under this Center: (EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R832414C001 Project 1 -- Pulmonary Metabolic Response
R832414C002 Endothelial Cell Responses to PM—In Vitro and In Vivo
R832414C003 Project 3 -- Inhalation Exposure Assessment of San Joaquin Valley Aerosol
R832414C004 Project 4 -- Transport and Fate Particles
R832414C005 Project 5 -- Architecture Development and Particle Deposition
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
Project Research Results
- Final Report
- 2010 Progress Report
- 2009 Progress Report
- 2007 Progress Report
- 2006 Progress Report
- Original Abstract
8 journal articles for this subproject
Main Center: R832414
128 publications for this center
64 journal articles for this center
