Grantee Research Project Results
Final Report: Recycling of Greenhouse Gases to Fuels & Chemicals
EPA Contract Number: EPD11046Title: Recycling of Greenhouse Gases to Fuels & Chemicals
Investigators: Molter, Trent
Small Business: Sustainable Innovations LLC
EPA Contact: Richards, April
Phase: I
Project Period: March 1, 2011 through August 31, 2011
Project Amount: $79,980
RFA: Small Business Innovation Research (SBIR) - Phase I (2011) RFA Text | Recipients Lists
Research Category: Small Business Innovation Research (SBIR) , SBIR - Greenhouse Gases
Description:
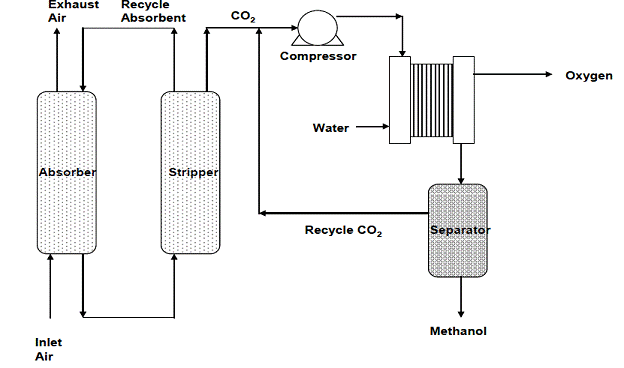
Summary/Accomplishments (Outputs/Outcomes):
|
Work Tasks |
Results |
| Task 1: Conversion of SOx and NOx to Useable Commodities | Modest increase in reaction temperature is beneficial to the production of hydrocarbon products. Controlling the ratio of reactants H2:CO2 affects the concentration of hydrocarbon products. |
| Task 2: Single Cell Hardware Fabrication | A dramatic increase in current density occurred the flow field of the baseline CO2 reduction stack was modified. The reaction rate was an order of magnitude faster. It is noteworthy that this stack was hydraulically tested, and exhibited no problems when its cathode was pressurized to 3,000 psi; therefore there was no obvious negative consequence of this modification in terms of the loss of the necessary structural integrity. |
| Task 3: Testing and Analytical Analysis | The gaseous exhaust of the CO2 reduction system was connected to the anode of a hydrogen detector cell. This cell was held at a constant voltage with a DC power supply which the electrical current drawn by the cell was monitored. The hydrogen in the stream was pumped across the membrane and was quantified by the current drawn by the cell. This allowed us to evaluate how much of the current in the EGGRS cell was used for CO2 reduction versus hydrogen generation. |
| Task 4: System Design Modifications – Advanced EGGRS Cell Design | Two major modifications were made to the system used to support EGGRS testing. Ports were added so that a precise volume of liquid could be added to the cathode of the cell. This modification allowed us to better quantify the products generated by the system. The second major modification was the addition of a gas manifold that allowed control of the flow rates of multiple low pressure gases. System tests showed 100% conversion of the cell current to hydrocarbon byproducts under nominal cell operating conditions. |
| Task 5: Top Level Design & LCA For Large Scale EGGRS System | The EGGRS will produce a range of commodity chemicals via the consumption of CO2 . The coupling of a CO2 emitter (conventional power generation plant) with an EGGRS and a low carbon footprint is feasible up to a giga watt scale. A 105 MW sized EGGRS will consume upwards of 1 million tonnes of CO2 over its lifetime (15 years). |
| Task 6: Techno-Economic Study of The EGGRS Technology | Upon completion of our techno-economic study we concluded that the EGGRS technology has a significant market position with respect to production of acetic acid and methanol. The Sankey and P&ID diagrams from Task 5 were incorporated into the techno-economic model. |
Conclusions:
- For nearly all hydrocarbon product scenarios from the EGGRS, the economics of the process appear to be favorable.
- When powered by renewable or nuclear energy sources, the EGGRS consumes more CO2 in a small fraction of a year than the CO2 emitted when building the EGGRS.
- Application scenarios and EGGRS' size significantly affect system economics.
- Hydrocarbon product global demands must be considered, as well as “unit value” of hydrocarbon product. In some cases, a single large EGGRS installation could meet the entire global demand of a particular hydrocarbon product (e.g., oxalic acid).
- The value of “carbon offsets” realized by the EGGRS is very low when compared to the value of product hydrocarbon and product oxygen.
- Even when powered by fossil fuel generated electricity, the EGGRS still can provide a lower cost method of producing most of the target hydrocarbons (than when produced in the traditional though in this case it would not be a carbon neutral process.
- Simpler hydrocarbon products result in much higher CO2 consumption.
Commercialization
Sustainable Innovations’ EGGRS system represents a viable commercial choice for recycling atmospheric pollutants into commodity chemicals. There has been real interest from stakeholders in:
- Atmospheric scrubbing of greenhouse gases.
- Recycling of greenhouse gases from large-scale emitters, including coal-fired power plants.
- Generation of fuels and commodity chemicals at small- to moderate-use sites.
The chemicals produced appear to be cost competitive with current commodity prices and have enormous market potential. The methanol market is, for example, growing and could provide annual revenues well in excess of $100 million after 5 years of sales.
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.
