Grantee Research Project Results
Final Report: High-Throughput Lung Damage and Inflammation Assessment of Polyaromatic Hydrocarbon Mixtures
EPA Grant Number: R840452Title: High-Throughput Lung Damage and Inflammation Assessment of Polyaromatic Hydrocarbon Mixtures
Investigators:
Institution:
EPA Project Officer:
Project Period: September 1, 2022 through May 9, 2025
Project Amount: $749,999
RFA: Development of Innovative Approaches to Assess the Toxicity of Chemical Mixtures Request for Applications (RFA) (2022) RFA Text | Recipients Lists
Research Category: Health Effects , Endocrine Disruptors , New Approach Methods (NAMs) , Human Health , Air , Safer Chemicals , Mixtures , Chemical Safety for Sustainability , CSS
Objective:
Our goal is to develop a new approach methodology (NAM) and apply this tool to reveal underlying connection between polyaromatic hydrocarbons (PAH) and PAH-derived SOA individually and as mixtures. We strive to reveal relationships between PAH and PAH SOA particulate matter (PM) properties and how they relate to biological effects (Fig 1). This project particularly focuses on biological effects associated with inhalation toxicity that leads to lung tissue injury and lung inflammation. Towards this overall goal, the approach of this project is to develop a NAM that can perform high-throughput dose-response analyses of PAH- and PAH SOA-mediated tissue injury and inflammation.
Figure 1. Overall Goal of this Project
Summary/Accomplishments (Outputs/Outcomes):
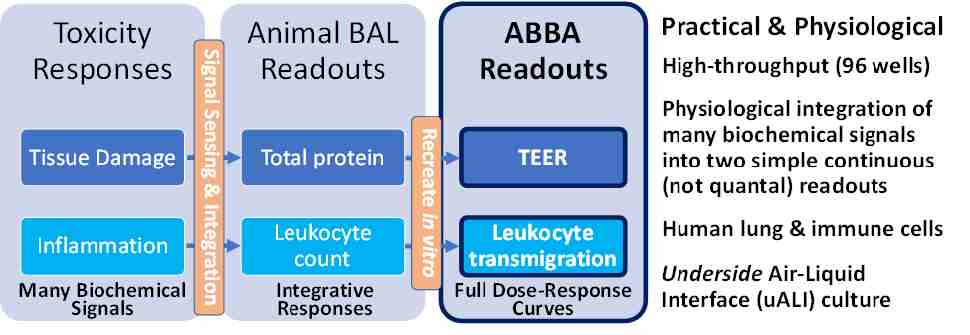
Concept of the NAM
The NAM concept is based on the need to predict human toxicity in a high-throughput manner using in vitro human cell culture-based models. Two key physiological inhalation toxicity measures obtained using animal models are: (i) lung tissue damage as measured for example through bronchoalveolar lavage (BAL) fluid total protein levels, and (ii) lung inflammation as measured for example through number of leukocytes that infiltrate the airways and are present in the BAL. Our NAM aims to obtain related readouts using a high-throughput, humans cell-based, in vitro model (Fig 2). The core platform is the Transwell-96-based air-blood barrier array (ABBA) described in more detail in Figure 3.
Figure 2. Basic Concept of the L-ABBA NAM
ABBA Quality Control Enhancements and Validation
Building on a Transwell-96-based ABBA we previously described (Viola et al. Adv Healthcare Mater 2021), we newly added leukocytes (“L”) on the blood side to produce the “L-ABBA” (Viola et al. APL Bioeng 2025) (Fig 3). Airway epithelial cells are seeded on the underside of Transwell membranes as previously described to enable physiological basal-to-apical transmigration of neutrophils through intercellular junctions. Neutrophils are loaded onto the upper endothelial compartment and chemoattractant is exposed from the lower epithelial compartment. Neutrophils transmigrate through the endothelium, the collagen-coated Transwell microporous membrane interstitium, and the ALI-differentiated epithelium to reach the chemoattractant.
An important component of the measurements is trans-epithelial electrical resistance (TEER) (Fig 3-5a). We validated QC methods focusing particularly on TEER that take into account variability arising from different batches of endothelial cells, different media, and different TEER electrodes. These methods and data are included in a recently published paper (Viola et al. APL Bioeng 2025).
Figure 3. Transwell-96 Leukocyte-laden Air-Blood Barrier Array (L-ABBA). 1) Underside flotation seeding of epithelial cells. 2) Endothelial cell seeding. 3) Underside ALI culture. 4)
Neutrophilic inflammation assay. 5a) Schematic of TEER. 5b) Co-culture (purple) synergistically enhances TEER compared to endothelial (green) or epithelial (orange) mono-cultures. [Viola et al. 2021, 2025]
As much of the details are included in [Viola et al. 2025], here we describe technical aspects and quality assurance results that have been obtained since the publication or are not included in the publication.
Enhanced TEER measurement procedures
Since the publication, to improve quality assurance for barrier property measurements, we have incorporated use of EVOM-Auto (WPI, Inc), an automated system that uses 8 electrodes to quickly measure TEER while the Transwell-96 plates are kept inside an incubator. This method has substantially reduced variability as well as environmental change stress on the cells.
L-ABBA alone is not sensitive enough to detect PAH immunotoxicity at the levels that they can be dissolved in aqueous solutions
PAHs are difficult to dissolve in aqueous solutions. We have found that, similar to many other in vitro tests, that PAH toxicity could not be detected within the concentrations that the PAH could be dissolved in aqueous solutions. For example, benzo[a]pyrene is soluble at only ~1 pg/mL directly in cell culture medium or even using 1% DMSO. We found that secondary organic aerosols (SOAs) derived from the PAHs are more water soluble and may dissolve to toxicity-inducing concentrations.
Macrophage effects
Having concluded that the L-ABBA as published in APL Bioeng 2025 has too low a sensitivity for PAH and PAH SOA toxicity sensing, we have started incorporating macrophage into the ABBA (Fig 4). The macrophage efforts build on past experience from the Ng lab in using mouse macrophage cell lines to evaluate aerosol responses and Takayama lab work on differentiation of human monocyte cell line (THP-1) towards macrophages. To maintain an all-human cell system and because THP-1 cells are used in some OECD test protocols, we worked to incorporate THP-1 derived macrophages into the ABBA.
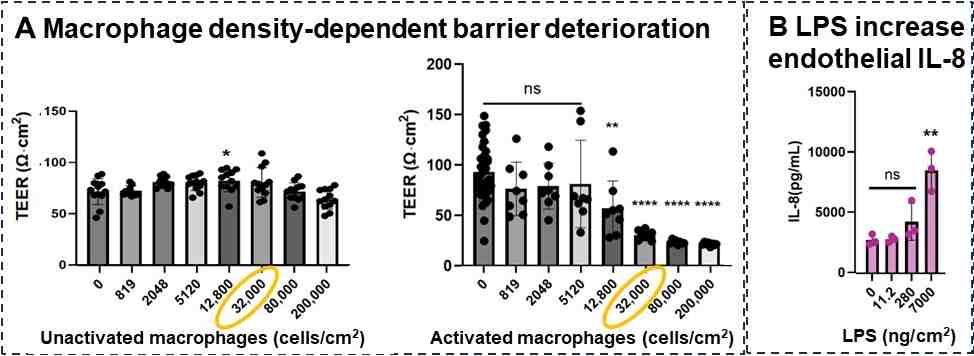
We have preliminary results of how activation of macrophage (in future they may be activated by PAH and PAH SOA) impacts the ABBA acutely in a macrophage cell number dependent manner as shown in Figure 4A. While the number macrophage per lung surface area varies between individuals and their state of health, a normal “healthy” lung is reported to have ~32,000 macrophage/cm2 although this count may account for not only airway macrophages but interstitial macrophage as well. Our preliminary data (Fig 4A) show that such normal as well as more pathological conditions with more macrophages are sufficient to induce a response in the ABBA. As a positive control, airway epithelial side stimulation with bacterial endotoxin (LPS) was observed to induce an inflammatory response that reaches the endothelial side across the Transwell membrane (Fig 4B). We did not get an opportunity to test PAH or PAH SOA in this system, but are optimistic this more physiological four cell type (macrophage, neutrophil, endothelial cell, airway epithelial cell) co-cultured platform will be more sensitive.
Figure 4. Acutely activated macrophages decrease barrier property within 24 hrs in a macrophage cell density dependent manner. (A) Unactivated macrophages have little effect but activated ones decrease TEER in a macrophage density dependent manner. (B) Airway side stimulation with bacterial endotoxin elicits an inflammatory cytokine response (IL-8) that manifests on the endothelial chamber.
Conclusions:
(a) We are highly optimistic about the technical effectiveness of the macrophage-incorporated L-ABBA (ML-ABBA) in terms of enhanced toxin sensitivity, although more validation is needed. In terms of economic feasibility, the ML-ABBA straddles the requirement for physiological relevant complexity with economic feasibility including throughput. Especially compared to lung-on-a-chip systems which can also have multiple cell types, the ML-ABBA has a significant economic feasibility advantage including higher throughput. White blood cell recruitment count and phenotype characterization, for example, is efficiently performed by interfacing with a flow cytometer with autosampling. Hence the ML-ABBA platform is technically effective and economically feasible by taking advantage of existing commercial technologies. The ML-ABBA is a static system and where stretch or flow is critical, lung-on-a-chip type systems may have an advantage in terms of technical effectiveness in some applications.
(b) Inhalation toxicity is traditionally performed using rodent models with functional readouts for air-blood barrier leakiness as quantified by protein levels in bronchoalveolar lavage fluid (BALF) and inflammation as quantified by white blood cell counts in the BALF. The current gold standard in vitro air-liquid interface (ALI) studies are generally focused on just the epithelium and often performed in lower throughput 24 well format. The L-ABBA and ML-ABBA are unique in co-culturing multiple cell types relevant to the lung and having in vivo-aligned functional outcomes not only of barrier property measurements such as TEER, but also counts of white blood cell recruitment from the endothelial to airway chamber. This opens the door for in vitro quantification of inhalation toxicity metrics for environmental toxicants and pollutants that can be directly related to functional in vivo metrics.
Journal Articles:
No journal articles submitted with this report: View all 3 publications for this projectProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.