Grantee Research Project Results
Final Report: Wetland and peroxide treated harmful algal blooms
EPA Grant Number: SU839870Title: Wetland and peroxide treated harmful algal blooms
Investigators: Vela, Jeseth Delgado
Institution: Howard University
EPA Project Officer: Page, Angela
Phase: I
Project Period: October 1, 2019 through September 30, 2020 (Extended to September 30, 2021)
Project Amount: $25,000
RFA: P3 Awards: A National Student Design Competition Focusing on People, Prosperity and the Planet (2019) RFA Text | Recipients Lists
Research Category: P3 Awards , P3 Challenge Area - Safe and Sustainable Water Resources
Objective:
Excessive nutrients can harm ecosystems and lead to the proliferation of algal blooms. Some of these bloom forming organisms are cyanobacteria that can produce toxins that harm wildlife and can impair drinking water sources. For example, in 2014 the city of Toledo’s drinking water treatment plant spent almost five million dollars to neutralize algal toxins[1], in spite of these investments the treatment plant was forced to shut down, causing significant public distrust of the plant. Given the high capital and social costs of treating toxins, improved strategies for mitigating toxin production are needed. Current strategies for eliminating cyanobacterial harmful algal blooms (CHABs) are focused on reducing nutrient emissions, both through point- and non-point sources. This objective of this research was to evaluate a process that combines nutrient emission reduction with treatment and mitigation of toxin production.
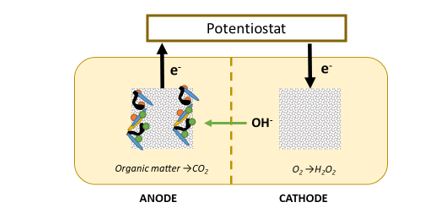
The team sought to understand and further develop a bioelectricochemical peroxide system to mitigate Cyanobacteria Harmful Algal Blooms (CHAB). The use of bioelectrochemical systems (BES) for hydrogen peroxide production is well established, with the majority of proposed applications being for wastewater treatment and disinfection (e.g. [2, 3]). The BES system is shown in Figure 1, organic matter is degraded by bacteria in the anode. The bacteria transfer the electrons from the degradation of organic matter via an electrode to the cathode. The cathode is open to the atmosphere, and the electrons are transferred to hydrogen peroxide. In theory, if enough carbon is available to bacteria, no external energy is required. However, a low voltage will increase production of H2O2. An advantage or this system is that unlike other BES systems the production of peroxide does not require any expensive catalyst.
Figure 1: BES system investigated in this project
The production of H2O2 is useful because it is a algaecide that does not have long-term detrimental effects on the environment [4, 5]. Ideally enough peroxide would be generated to mitigate CHABs, however in the case where blooms are at peak, a low amount of external energy can be supplied if needed to produce higher amounts of hydrogen peroxide. One advantage of this system is that it can also provide a distributed, continuous, source of peroxide throughout a water body.
The focus of the research was twofold: (1) demonstrate the dose required of peroxide to effectively kill Microcystis aeruginosa and (2) demonstrate peroxide production using a bioelectrical system and compare this bioelectrical peroxide system to existing mitigation technologies with Life Cycle Assessment techniques. The research also sought to give undergraduate students from diverse disciplinary backgrounds an opportunity to demonstrate P3 principles. Examples of managing people, prosperity, and planet were incorporated into Introduction to Environmental Engineering Course and the interdisciplinary Howard University Water Environment Association were also exposed to sustainable water management. The research project was also integrated into an Undergraduate Research Course. The project was converted to a case study that shows the social, environmental, technical, and economic factors that need consideration for sustainable engineered solutions. Engineering students benefited from seeing the variety of disciplinary backgrounds that are needed to provide sustainable and technical solutions to environmental problems.
Summary/Accomplishments (Outputs/Outcomes):
The primary performers of the project were 5 undergraduate students 4 Civil and Environmental Engineering students and 1 Sociology/Environmental Studies major. Students had diverse interests in environmental engineering and science and worked together to complete project objectives. The laboratory was shut down on March 16th, 2020 due to the COVID-19 pandemic. Howard University was 100% virtual from March 16th 2020-August 23rd 2021, during the vast majority of the project period. Prior the pandemic, the team began building the BES system and developing methods for measuring hydrogen peroxide colorimetrically with a titanium oxide and sulfuric acid incubation. The team developed a protocol for measuring the impact of light and H2O2 on Microsystis aeruginosa. These aspects of the project could not be completed due to the pandemic. The focus of the project and this report was therefore comparing the bioelectrical peroxide system to existing mitigation technologies with Life Cycle Assessment.
The student developed a case study for a Life Cycle Comparison. The LCA was conducted assuming the need for an active hydrogen peroxide does of 2 mg/L for 5 hours of treatment based on prior literature [6]. The two scenarios that were compared were using manufactured hydrogen peroxide that is transported by boat to the Gulf of Mexico off the Florida Gulf Coast. The second scenario was using a bioelectrical peroxide production system. The LCA was developed using OpenLCA.
The student team made assumptions about the BES system using literature of similar BES hydrogen peroxide production using a different application [4, 5]. The impact categories included for the LCA were ozone depletion, land use, particulate matter, photochemical ozone formation (human health), resource use (fossils, minerals & metals), water use, eutrophication (marine, freshwater, terrestrial), ecotoxicity (freshwater), human toxicity (non- & cancer), ionising radiation, acidification, and climate change (biogenic, fossil, land use & land use change). For the BES system the team conducted research on the production of the anode and cathode and determined the key production aspects that effect the environmental impact categories. Students utilized preexisting EF Secondary Data set with the Environmental Footprint (Mid-point indicator) and OpenLCIA Impact Assessment to convert inventory data to impact categories.
The anode was assumed to be graphite felt produced via a pyrolysis process [7, 8]. Environmental inputs for pyrolysis were determined using the literature and is shown in Table 1. The team evaluated the key flow aspects that resulted in detrimental environmental impacts. Bleaching eucalyptus pulp had the highest effect on most of the impact categories: ozone depletion, land use, photochemical ozone formation, and eutrophication. Climate change was most effected by diesel use for the production process.
| Cellulose Production for Anode | Aluminum for cathode |
| Bleached Kraft Pulp | Bauxite |
| Chlorine Dioxide | Heat transfer oil |
| Diesel production | Transport |
| Electricity | Sodium hydroxide caustic |
| Eucalyptus forestry | Electricity |
| Sulfur dioxide | Carbon fiber |
| Sulfuric acid | Transport |
| Transport | |
| Water | |
| Water |
Table 1: Inputs for anode and cathode
The cathode was assumed to be fabricated aluminum made mined in bauxite. The inputs to the LCA included the mining process and the production process to make the aluminum cathode (Table 1). Heat transfer fluid oil for heat transfer needed during the conversion of bauxite to aluminum had by far the highest environmental impact for land use, climate change, and ozone depletion.
The team found that cathode environmental impacts exceeded anode environmental impacts by an order of magnitude for land use and climate change impact categories. They concluded that so evaluating alternative cathode materials will have an outsized impact on the environmental sustainability of the BES process. Similarly, diesel for the boat transport had a high impact on the baseline scenario of hydrogen peroxide dissemination. The project resulted in the training of several students in research methods and sustainable water technologies. The students learned how to navigate uncertainty in research and develop different baseline scenarios for environmental impact assessment.
Supplemental Keywords:
Water, chemicals, effluent, bacteria, ecosystem, aquatic, clean technologies, innovative technology, remediation, restoration, cost-benefit, socio-economic, biology, social science, engineeringProgress and Final Reports:
Original AbstractThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.