Grantee Research Project Results
2014 Progress Report: Lessons from Nature - Synthetic Humic Acid Materials for Improved Water Purification
EPA Grant Number: SU835545Title: Lessons from Nature - Synthetic Humic Acid Materials for Improved Water Purification
Investigators: Burkhardt, Cindy A , Webster, Francis , Gerard, Angela , Kraft, Grace , Cardenas, James , Hayes, Spencer , Gammarino, Ian
Institution: Radford University
EPA Project Officer: Hahn, Intaek
Phase: II
Project Period: August 15, 2013 through August 14, 2015
Project Period Covered by this Report: August 15, 2013 through August 14,2014
Project Amount: $89,975
RFA: P3 Awards: A National Student Design Competition for Sustainability Focusing on People, Prosperity and the Planet - Phase 2 (2013) Recipients Lists
Research Category: Pollution Prevention/Sustainable Development , P3 Awards , P3 Challenge Area - Safe and Sustainable Water Resources , P3 Challenge Area - Chemical Safety , Sustainable and Healthy Communities
Objective:
Due to population increases and rapid industrialization around the world, water quality and the ability to ensure the availability of reliable clean drinking water will be an increasingly important global concern. The lack of clean water is most keenly felt in developing countries where more expensive water purification technologies are not available. The need for clean water has spurred a variety of research efforts related to the development of new methods and materials for water purification. Our project focused on the development of an inexpensive, multi-functional adsorbent material that will improve existing sand filtration technology to better remove a wide range of water contaminants, including arsenic, other heavy metals, and organic contaminants. The adsorbent is based on our recent discovery of a novel synthetic humic acid-like carbon material (sHAC) that is economical, easy to prepare, and based on renewable starting materials. The overarching goal of this project is to mimic the humic acid found in nature, a natural polyelectrolyte that easily binds to minerals and serves as an excellent chelator of metals in the environment
In our Phase I project, we were able to synthesize and fully characterize a sHAC material with heterogeneous, but defined, chemical structure using renewable starting materials, including sugar and sugar alcohol sources, and a low temperature one-pot synthesis. We prepared three composite materials, including sHAC/sand, sHAC/iron-coated sand, and sHAC/magnetic iron nanoparticles, and fully characterize these materials, which were used to remove arsenic, heavy metals, and a model organic dye in controlled water systems. The Phase II project focused on the investigation whether the technology can be scaled to produce the larger carbon quantities needed to make a real impact on pollution reduction and to test our adsorbent material under the more “real world” conditions. Each challenge is outlined below with a summary of the outcomes achieved over the last year.
Progress Summary:
Challenge 1. Develop the technology to increase the carbon batch production size at least 10 times while reducing the processing times
While the small scale preparation of a sHAC material (0.5 kg carbon gel, 2-3% carbon) can easily be prepared using many simple sugars and sulfuric acid, the use of this material on a larger scale depends on the production of larger quantities of the nano-carbon material. To test the concept of continuous carbon production, a continuously stirred tank reactor (CSTR) was designed and constructed according to the generic schematic diagram shown in Figure 1 below.
Figure 1. Schematic design and working reactor for the CSTR reactor used for the continuous production of sHAC.
In general, a reactor temperature of 175oC was maintained with a 16.5:1 (vol/vol; 15.5 mol/mol) ratio of acid to glycerol. The glycerol inflow was 1.0 mL/min and a post-reaction removal pump was used with a flow rate of approximately 13 mL/min. A one liter reaction flask with magnetic stirrer was used and a teflon tubing coil (12.5’,1/4” diameter) in ice was used as the heat exchanger before in-line dilution and deposition in the settling tanks (not shown in Figure 1).
While the system generally worked as designed, there were a number of design problems that were corrected including:
- The magnetic stirrer used in the original design was not adequate and the stirrer often stopped during synthesis leading to solid formation in the reactor. A completely new reaction vessel (one liter glass design with six-port Teflon top and a more powerful heating mantle system) was designed with overhead stirrer capability which resolved this problem.
- A balance was placed under the reactor and used to monitor the reactor mass. Pump flow adjustments were continuously made to maintain constant mass during the reaction.
- An in-line dilution pump (13:1 vol/vol cold deionized water/carbon slurry) was added to provide consistent dilution before the reaction mixture reached the settling tank.
With these changes, the reactor could be run for hours and produced a carbon product that was both textually and chemically identical to the material prepared in the batch reactions. Overall, the restults showed that the CSTR reactor worked as planned and proved the concept that continuous production of carbon material could be achieved.
Challenge 2. Scale the sand coating process to kilogram amounts quantities needed for larger scale filtration projects.
As in the first challenge, the widespread use of our adsorbent for pollution remediation will depend on the scale-up of adsorbent production. Previous work had shown that the adsorbent properties are very dependent on exactly how the material was prepared (water evaporation time, temperature, and ageing time) so the challenge of the Phase II project was to evaluate the importance of these parameters on material scale up.
Improved Carbon Coating Techniques
Scaling up the amount of carbon coated sand produced proved to be more difficult than anticipated and at least six months was devoted to the design and testing of methods to produce larger quantities of coated sand. Many designs were attempted and later discarded including vibration coating techniques, rotating drum coating systems, and various stirred oven designs. Attempts to simply increase the amount of starting material used often resulted in incompletely coated sand and in a two phase final product consisting of dried carbon and dried sand. The amount of liquid remaining during the drying procedure was found to be critical and too much residual water resulted in two solid phases with no sand coated.
To overcome these problems, a new method was developed and relied on the use of a minimum amount of water in which the carbon gel / sand mixture was first was prepared with a grout-like consistency before drying. The mass of carbon gel was carefully monitored and the amount needed was found to depend on the particle size of the sand used which was then carefully controlled. For a particle size of 105-180 micron sea sand for example, a maximum of 6.25 grams of gel per 40 grams of sand were needed to give the required consistency. The coated sand was then heated to 70oC in an air oven with occasional stirring, washed several times, and then dried again at the same temperature to give the final product.
A large scale oven with stirrer was designed and constructed for the preparation of up to five kilograms of the carbon coated sand. While work is still ongoing to improve the performance of the design, clearly much larger amounts can be made with the new carbon coating method and illustrates the proof of concept that large amounts could be prepared at reasonably low temperatures. While adsorption tests are on-going the evaluate the properties of the large batch sand samples, the performance so far has been similar to that seen for the small laboratory bench size batch studies.
Improved Carbon/Iron Composites
Scaling up the amount of goethite / carbon composite material produced was in many ways more challenging than the carbon coated sand. It was finally determined that the method previously used in Phase I to coat sand with a mixture of goethite/carbon was impractical and that new methods were needed to increase the amount coated sand produced. To improve the process for producing large amounts, a ferrihydrite / carbon gel was first synthesized which was then used to coat the sand.
Figure 2. Simple oven based coating system for the preparation of up to 10kg of carbon coated sand.
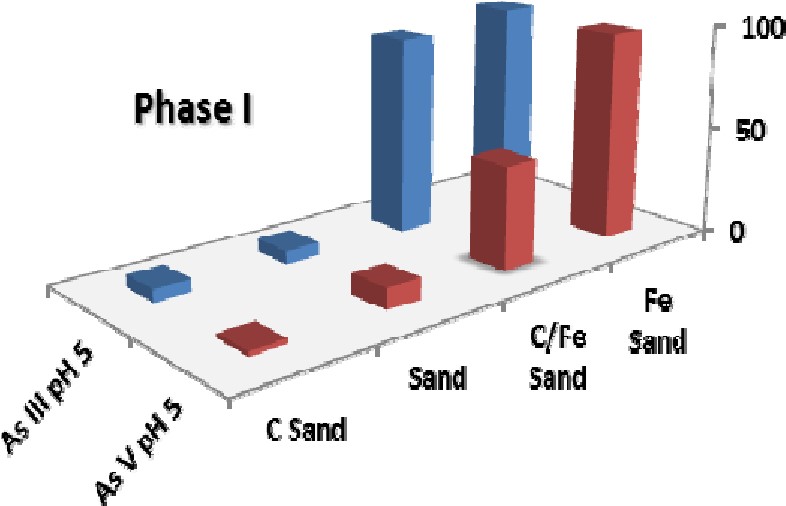
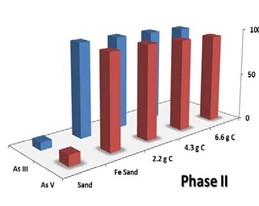
In a typical synthesis, fixed amounts of carbon gel were added to a ferric chloride solution (for example, 4.4 g gel was added to 70mL of 0.062M ferric chloride in a typical synthesis) and the pH slowly raised to a pH of 7.0 with extensive mixing. The neutral pH ensured that ferrihydrite was formed instead of the goethite previously used in Phase I. This gel was then washed and then added to sand in the same proportions as was used with carbon coating with heating in an oven at 70oC for 24 hours with occasional stirring. After extensive washing, this material was dried to form sHAC / ferrihydrite coated sand. While extensive testing of the material is currently underway, the material was superior to the goethite/carbon coated sand previously produced with respect to the adsorption of arsenic. Figure 3 shows a comparison of goethite coated sand and ferrihydrite coated sand under the similar adsorption conditions showing the improved ability of the ferrithydrite/carbon coated sand to remove both arsenic (III) and arsenic (V) regardless of the carbon loading.
Figure 3. Adsorption results for arsenic removal by iron coated sand for goethite coated sand (Phase I) and ferrihydrite coated sand (Phase II). Both adsorption tests were performed using approximately 12mL of 1.0ppm As(III) and As(V) and 0.250g of coated sand with 24 hours equilibration.
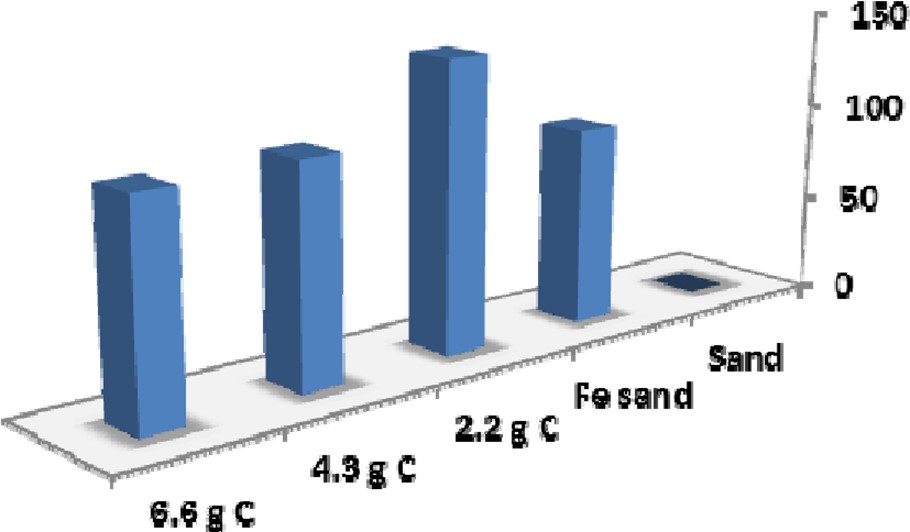
In addition to heavy metals, arsenic, and methylene blue tested proposed in the Phase I and II proposals, carbon / ferrihydrite coated sand was also tested for the ability to remove phosphate, a common water pollutant which can be toxic to organisms in high amounts. Figure 4 shows a plot of the phosphate removed (µg / gram sand) for the ferrihydite / carbon coated sand at increasing carbon loading levels. The results indicated removal levels that were similar to iron coated sand and relatively independent of the carbon loading.
Figure 4. Adsorption results for phosphate removal by ferrihydrite coated sand (µg / gram sand). For these tests, 0.250g of coated sand was added to 12mL of 104µM PO43- (Na2HPO4) at a pH = 7.0
Challenge 3. Test adsorbent characteristics under chemical conditions found under realistic conditions where the contaminant level is much lower and the water has more representative water chemistry. This will be done through the development and automation of the rapid small scale column test (RSSCT) system and the use of naturally contaminated waters.
To test our adsorbents under flow conditions, the Rapid Small Scale Column Test (RSSCT) was developed and mathematical models used to characterize adsorption behavior. For this test, a small column size was used to predict the results for pilot or full scale column systems. If the hydrodynamic characteristics of the small and large columns are similar, breakthrough curves are expected to be similar and pilot or full scale columns can be designed using the results of the RSSCT. The advantage of this approach is the much lower test cost for required materials, shorter time for analysis, and the much lower amount of water needed to test potential adsorbents.
The characteristics of the adsorption process were modelled using the Thomas model which is widely used for modeling adsorption in column studies. It assumes a Langmuir adsorption model with second order reversible kinetics and can be described by the following equation.
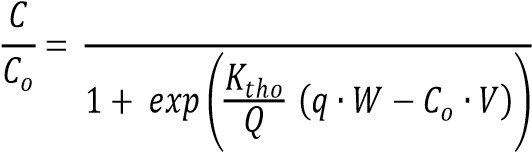
where C is the concentration of the adsorbate, Co is the feed stream concentration, Ktho is the Thomas rate constant, q is the amount adsorbed in equilibrium, W is the mass of adsorbent, V is the throughput volume and Q is the volumetric flow rate.
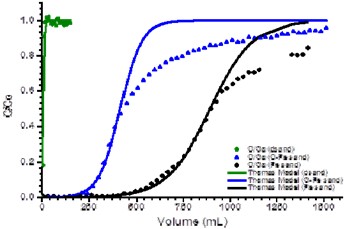
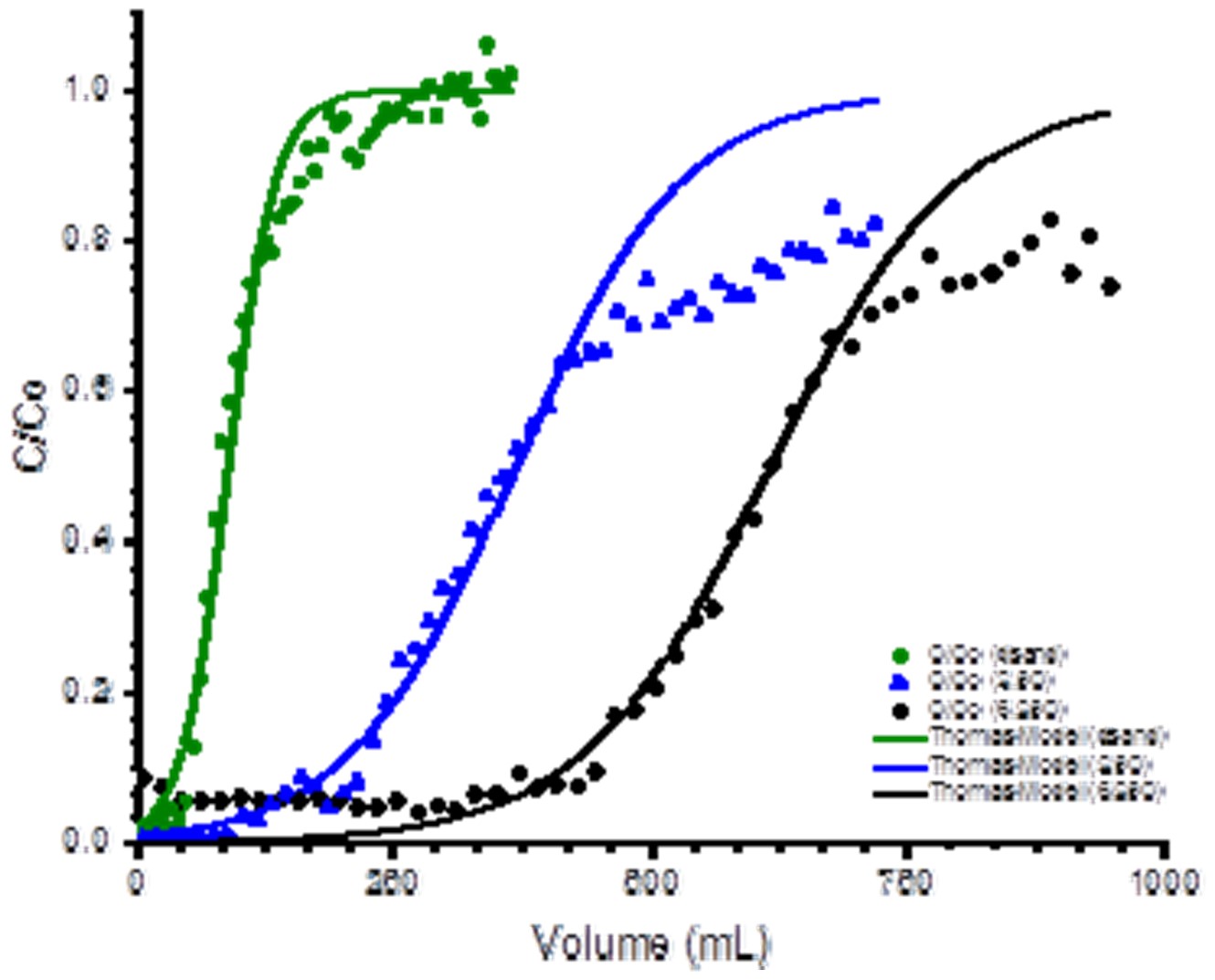
Figure 5 shows a plot of C/Co versus the elution volume for the removal of arsenic (III) using ferridhydrite / carbon coated sand.
Figure 5. Plot of C/Co versus the elution volume for uncoated sand (dsand), ferridhydrite coated sand (Fe sand) and carbon/ferrihydrite coated sand (C-Fe sand) showing the best fit of the elution profile data to a C/Co of 0.50 using the Thomas model.
The column used in this study was 0.7cm in diameter with 105-180 micron sand particle size and a flow rate of approximately 3 mL/min. A mass of 5.2 grams of coated sand (3.4 mL bed volume) was transferred to the column in a water slurry form and washed with water for one hour before use. Adsorbent solutions were prepared at a concentration of approximately 1.0 ppm at a pH of 5. Aliquots were periodically taken and analyzed by ICP.
Results showed that the Thomas model could accurately fit the data up to approximately 50% total elution, but the model significantly deviated from experimental data after at later times and underestimated the total adsorbed amount of arsenic (III) adsorbed. Since water filtration systems are generally concerned with adsorbent breakthrough to values exceeding maximum allowable levels, the model can still be used to characterize and evaluate coating performance. In the case above, the ferrihydrite/carbon coated system was substantially better than uncoated sand but less effective than that seen for ferrihydrite coated sand alone for arsenic (III) adsorption. This clearly demonstrates a better evaluation of performance under more realistic conditions than observed when simple batch studies are used.
Similar results were seen for copper adsorption on carbon coated sand as shown in Figure 6 below.
Figure 6. Plot of C/Co versus the elution volume for uncoated sand (dsand), and carbon coated sand showing the best fit of the elution data to a C/Co of 0.50 using the Thomas model.
The Thomas model again fit the data well only up to a C/Co value of approximately 0.5 with significant deviations after that. There was a direct correlation between the breakthrough volume and the amount of carbon loaded on the sand indicating that column performance could be improved simply through the addition of more carbon gel when making the coating.
A second component of “real world testing” for the Phase II project was the evaluation of our adsorbent material to remove arsenic from environmental water samples taken from the out-flow of the 85 year old abandoned arsenic mine in Floyd County, Virginia. Access to the site is currently being negotiated and testing will hopefully begin this summer.
Challenge 4. Partner with industry having expertise in the testing of adsorbent materials on a larger scale needed for pollutant remediation.
The fourth and final challenge of the Phase II project was to partner with a company (Filterra Incorporated in Richmond, Virginia) to test our adsorbents using the typical conditions required for storm water remediation applications. Unfortunately, Filterra was purchased by Contech Engineered Solutions LLC last year and a reorganization of the company occurred. The new management was no longer interested in partnering with us on this project, so considerable time was spent during the last six months to find another industrial partner interested in exploring the potential commercial uses for our carbon based materials. During this evaluation process, we discovered that while Filterra could indeed test our material for storm water applications, they were slated to perform similar column laboratory tests that were currently being done done by students at Radford. This led to an expansion of the project scope to also include other potential industrial partners to help us explore potential new uses for our nano-carbon material. Primarily, we were interested in novel uses where added functionality could be integrated to existing systems by direct nanoparticle addition during synthesis or through the use of layer-by-layer techniques which we have developed at Radford.
To this end, we have recently signed a non-disclosure agreement with Nanosonic Incorporated, a local company interested in partnering with us to investigate the use of our renewable nanocarbon material to add functionality to polymer systems. The hope is that we can use sustainable technology to improve existing membrane filtration systems through the addition of well controlled functionality in a nanostructured carbon matrix. We have had two productive meetings with the science team at Nanosonic this semester, have decided on project of mutual interest, and are now developing statements of work that should be completed in the next several weeks.
Future Activities:
The objective of balancing the elements of people, prosperity, and the planet was paramount in all aspects of the Phase II research. To reduce the cost of production, an adsorbent for water purification was prepared using simple one-pot methods with bio-renewable sugars and other readily available starting materials. Significant progress was made in achieving the four major goals of the Phase II project. A CSTR reactor was designed and tested for the continuous production of our renewable nano-carbon material, a 5-10kg scale coating system was designed and tested for the coating of large quantities of our sand adsorbent, column tests were improved and models tested for flow adsorption studies, and new industrial partners were identified to test novel uses for our novel carbon material derived from sustainable starting materials. Progress toward sustainability will happen when scientists learn to design materials using renewable resources, require less energy to prepare them, and generate fewer by-products with directed uses for each.
Journal Articles:
No journal articles submitted with this report: View all 1 publications for this projectSupplemental Keywords:
Water purification technologies, water treatment, water filtration, coated-sand adsorbents, synthetic humic acidProgress and Final Reports:
Original AbstractP3 Phase I:
Lessons from Nature - Synthetic Humic Acid Materials for Improved Water Purification | Final ReportThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.