Grantee Research Project Results
Final Report: A Solar-powered, Sustainable, Hybrid Electrodialysis/Reverse Osmosis Desalination System for Rural Areas of Developing Countries
EPA Grant Number: SU835310Title: A Solar-powered, Sustainable, Hybrid Electrodialysis/Reverse Osmosis Desalination System for Rural Areas of Developing Countries
Investigators: Nossoni, Goli , Abulencia, James Patrick , Gallardo, Susan , Gallardo, Ronald , Martinez, Helmer , Qasim, Mohammad , Markes, Kiley , Piazza, Alessandra
Institution: Manhattan College , De La Salle University-Manila
EPA Project Officer: Hahn, Intaek
Phase: I
Project Period: August 15, 2012 through August 14, 2013
Project Amount: $14,950
RFA: P3 Awards: A National Student Design Competition for Sustainability Focusing on People, Prosperity and the Planet (2012) RFA Text | Recipients Lists
Research Category: Pollution Prevention/Sustainable Development , P3 Challenge Area - Safe and Sustainable Water Resources , P3 Awards , Sustainable and Healthy Communities
Objective:
The proposed project of the Manhattan College and De La Salle University Research Team is to create a hybrid ED/RO desalination method using concrete as the permeable membrane. Concrete can be produced locally and with recycled material so it is low cost, and the energy required for this system can be supplied by a solar panel. A successful design will have significant impact on providing clean, potable water for developing nations, especially those proximal to oceans and seas.
Summary/Accomplishments (Outputs/Outcomes):
The suitability of the parameters was judged based on the conductivity of the solution in the desalination chamber, which was used as a surrogate measure for the salinity. It is noteworthy to mention that since concrete pores inherently contain a high concentration of mobile ions, it is possible that unwanted ions may infiltrate to the desalination chamber and increase the conductivity. However, increased conductivity resulting from leaching would produce a bias on the conservative side for assessing ion concentration in the desalination chamber.
-
Pervious concrete performs better as a membrane, compared to normal concrete.
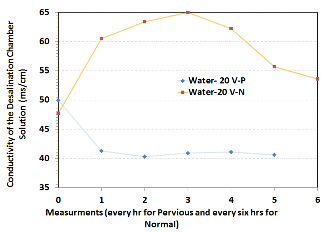
Normal concrete and pervious concrete were compared in their ability to remove ions (as measured by conductivity in the desalination chamber). Figure 1 shows the efficacy of both samples. Normal concrete actually increased the conductivity from 47 to 53 ms/cm over 36 hours, while pervious concrete allowed conductivity to decrease from 50 to 41 ms/cm over only 6 hours, which corresponds to an almost 18% reduction.
Figure 1: Conductivity measurements comparing normal and pervious concrete
-
Lime water performs better as the solution in the anodic chamber, compared to water.
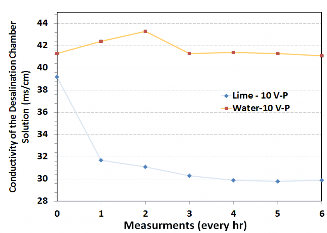
Lime water and distilled water were compared in their ability to remove ions (as measured by conductivity in the desalination chamber). Distilled water was used in this set of experiments to avoid introducing any bias to the conductivity reading. Figure 2 shows that lime water reduced the conductivity from 39.5 to 29 ms/cm, a 27% reduction in six hours, while using distilled water reduced the conductivity by only 1%.
Figure 2: Conductivity measurements comparing lime water and distilled water in the anodic chamber.
-
An electric potential of 10V is better in removing the ions in the desalination chamber compared to 20V.
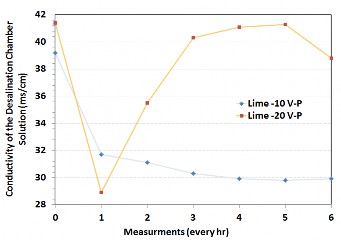
The magnitude of the electric potential between the two electrodes was compared in their ability to remove ions (as measured by conductivity in the desalination chamber). Figure 3 shows that the conductivity of the solution reduced by 33% over the first hour when 20 V was used, but then increased. This is probably due to the greater mobility of ions under a high electric field, causing liberation of ions originating from the concrete. Use of 10 V produced better results and the conductivity of the solution decreased by 27%. Additionally, the smaller potential translates into a lower energy requirement for operation. Assuming four chambers arranged in series, a 10 watt solar panel working for an average of 4 hours per day would be sufficient to power the laboratory scale test set up.
Figure 3: Conductivity measurements comparing a 10V and 20 V electric potential
-
Removing the effect of endogenous ions from concrete also decreases conductivity in the desalination chamber.
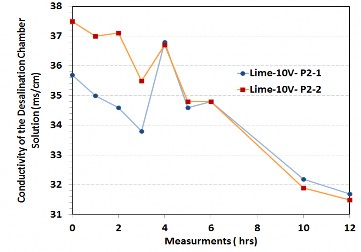
As stated earlier, concrete pores inherently contain a high concentration of mobile ions. To minimize the effect of these endogenous ions, the size of the desalination chamber was increased such that the concrete volume is small compared to the solution volume. Additionally, the concrete slabs were cured while submerged in water, to allow free ions to leach out prior to placement in the desalination chamber. Figure 4 shows two parallel tests indicating a reduction of conductivity over 12 hours. The results from the two tests are in very good agreement over time.
Figure 4: Parallel tests using concrete with minimal endogenous ions.
Journal Articles:
No journal articles submitted with this report: View all 1 publications for this projectSupplemental Keywords:
Desalination, Concrete, Conductivity, Zeolite, Ion exchange, Electrodialysis,
The perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.