Grantee Research Project Results
Final Report: Carbonation of Mine Water to Increase Limestone Dissolution and Alkalinity Generation
EPA Contract Number: 68HERC20C0032Title: Carbonation of Mine Water to Increase Limestone Dissolution and Alkalinity Generation
Investigators: Hedin, Robert
Small Business: Hedin Environmental
EPA Contact: Richards, April
Phase: I
Project Period: March 1, 2020 through August 31, 2020
Project Amount: $99,523
RFA: Small Business Innovation Research (SBIR) - Phase I (2020) RFA Text | Recipients Lists
Research Category: Small Business Innovation Research (SBIR) , SBIR - Land Revitalization , SBIR - Mining and Mine Waste Management
Description:
Mine water is commonly characterized as "acid" because of excess acidity (e.g. low pH) and the ability of dissolved metals (e.g. Fe, Al, and Mn) to generate acidity during precipitation reactions. For example, one mole of dissolved Fe(II) generates two moles of acidity after oxidation and hydrolysis:
Fe(II) + 0.25O2 + 2.5H2O --> Fe(OH)3 + 2H+
One of the primary goals of mine water treatment is the generation of alkalinity (HCO3- or OH-) to neutralize the potential acidity from metal precipitation. Mine waters can generate hundreds of mg/L of acidity which requires equal concentrations of alkalinity to neutralize. High concentrations of alkalinity are easily generated by soluble bases such as NaOH and Ca(OH)2. However, limestone treatment, the most cost-effective method to generate alkalinity in mine water, is subject to thermodynamic calcite solubility controls that restrict the generation of high concentrations of alkalinity. One factor controlling calcite solubility is dissolved CO2, or carbonic acid, concentrations:
CO2(g) + H2O --> H2CO3 + CaCO3(s) --> Ca+2 + 2HCO3-
Current limestone systems rely on natural CO2 concentrations in mine water to dissolve calcite and generate alkalinity. This research aimed to increase calcite dissolution and alkalinity generation in limestone treatment systems by carbonation.
To develop mine water carbonation technologies, two experiments were conducted: 1) carbonation of two pilot scale limestone systems, and 2) carbonation of a full scale, operational limestone treatment system.
For the pilot scale systems, two, 600 ft3 roll off containers were filled with 4 feet of limestone (30 tons) and covered with 6 inches of compost to keep the system anoxic via high biological oxygen demand. CO2 gas was introduced into the roll off containers via "in-bed" and "in-line" technologies. In-bed technologies involved injecting CO2 underneath the limestone. Four in-bed lines of porous tubing were installed in each container. In-line technologies involved injecting CO2 into the mine water pipeline before the mine water reached the roll off containers. This method involved the use of a standalone carbonator device.
The full-scale treatment system carbonation experiment was conducted at the Howe Bridge treatment system (Jefferson County, PA). Howe Bridge is a full-scale operational limestone system constructed in 1990 and rehabilitated in 2020. The Howe Bridge system consists of a buried bed of limestone (300 tons) followed by settling ponds and wetlands. The system treats 20 gpm of mine water containing 128 mg/L Fe and 20 mg/L Mn. Howe Bridge was chosen because the limestone bed does not generate sufficient alkalinity to neutralize acidity generated from Fe and Mn precipitation in the settling ponds/wetlands. Consequently, while the system decreases metal concentrations, the effluent of the ponds/wetlands is pH 3 to 4.
Experiments at Howe Bridge involved retrofitting the underperforming limestone system with carbonation technologies developed from the pilot scale experiments. At Howe Bridge, CO2 was injected via in-bed and in-line technologies.
During every site visit to the pilot scale and full-scale systems, gas flow rates, water flow rates, and influent/effluent pH, alkalinity, temperature, and conductivity were measured. Periodically, influent/effluent water samples were collected for laboratory analysis of acidity and dissolved ions. Geochemical models of the systems were constructed using PHREEQC software.
Summary/Accomplishments (Outputs/Outcomes):
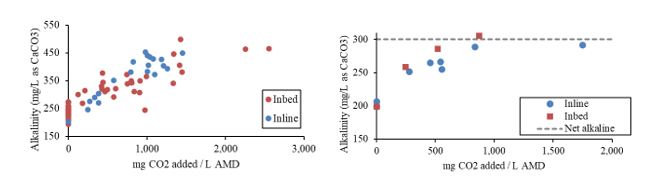
In both experiments, the carbonation of mine water resulted in increased effluent alkalinity concentrations. Furthermore, there was a strong relationship between the amount of CO2 added (as mg CO2 added / L mine water) and effluent alkalinity concentrations at both testing locations. Significantly, the full-scale limestone systems produced a net alkaline effluent (e.g. sufficient alkalinity to neutralize all potential acidity) for the first time in the system's 30-year history. Both the in-bed and in-line carbonation technologies developed had similar relationships between CO2 addition and effluent alkalinity (Figure 1).
Figure 1. The relationships between CO2 addition concentrations and effluent alkalinity concentrations from the pilot scale experiments (left) and the full-scale treatment system experiments (right). The relationships between CO2 added and effluent alkalinity concentrations are similar between in-bed and in-line CO2 addition technologies. The alkalinity concentrations required for net alkaline conditions at the full-scale system is noted.
Total inorganic carbon (TIC) mass balances were constructed to calculate the CO2 transfer efficiency (i.e. % C from CO2 gas that was dissolved). Missing carbon was assumed to be outgassed or never dissolved and wasted. Mass balances show that an average of 79% to 85% of CO2 added via the in-bed and in-line carbonation technologies, respectively, at the pilot scale systems was dissolved. The remaining 21% to 15% was lost to the atmosphere. While this data suggests that the pilot scale carbonation experiments dissolved most of the CO2 gas added, the technology can still be optimized to ensure 100% of CO2 is hydrolyzed and reactive and there is no opportunity for outgassing.
Kinetic studies were conducted where mine water flow rate and theoretical retention time were varied while CO2 injection rate was held constant. The experiments established that carbonation decreased the theoretical retention time for maximum alkalinity generation from 10-12 hours to 3-4 hours. This result indicates the CO2 enhanced limestone treatment systems could be substantially smaller than current limestone systems which will decrease capital costs and system footprint.
The primary cost component for the limestone+CO2 technology is the cost of CO2. Calculations from the pilot scale experiments suggest that the cost to generate alkalinity from the limestone+CO2 technology is similar to the costs currently realized from the purchase of lime slurry at the pilot site. The cost of the limestone+CO2 technology can be decreased with optimization of gas transfer and calcite dissolution processes. If CO2 capture technologies are developed that increase the supply of commercial CO2, subsequent reductions in CO2 cost could substantially improve the economics of the technology.
Conclusions:
This project successfully developed and installed carbonation technology to increase alkalinity generation from both pilot scale and full-scale limestone treatment systems. In-bed carbonation technologies, CO2 injected directly into the limestone bed, and in-line carbonation technologies, CO2 injected into mine water before entering the limestone bed, were developed and installed. Experiments varying CO2 and mine water flow rates produced strong relationships between the concentration of CO2 added and concentration of alkalinity in the pilot system's effluent. Additionally, the carbonation retrofit of the full-scale treatment system resulted in sufficient alkalinity generation to neutralize all potential acidity for the first time in 30 years of operation.
In the pilot scale experiments, both in-bed and in-line technologies were approximately 80% efficient at transferring gaseous CO2 to the dissolved phase with the remainder outgassed and wasted. Future research will work to maximize CO2 dissolution efficiency and thus lessen reagent cost.
When fully developed, the limestone+CO2 technology will potentially generate two streams of income: 1) licensing fees associated with the novel technology; and 2) fees from CO2 sales. Further technology development will yield propriety technologies associated with the carbonation methods/devices and improvements to the carbonation process. This technology is novel and will be attractive to engineering companies that specialize in mine water treatment systems. The technology will be licensed to engineering firms in the US and worldwide. The limestone+CO2 technology will also create a new market for CO2 suppliers. Licenses will be developed with CO2 suppliers that provide exclusive rights for CO2 sales associated with use of the technology.
SBIR Phase II:
Carbonation of Mine Water to Increase Limestone Dissolution and Alkalinity Generation | Final ReportThe perspectives, information and conclusions conveyed in research project abstracts, progress reports, final reports, journal abstracts and journal publications convey the viewpoints of the principal investigator and may not represent the views and policies of ORD and EPA. Conclusions drawn by the principal investigators have not been reviewed by the Agency.