You are here:
PRODUCTION OF NATURAL PLASTICS IN WASTEWATER TREATMENT
Impact/Purpose:
Commercialize a technology to produce biodegradable plastics concurrent with wastewater treatment.
Description:
Site Sourcing
Locating our research on site at a wastewater plant proved less reasonable than simply shipping biosolids from the site to our lab. By not locating at one particular site, we were able to experiment with biosolids from several wastewater treatment plants and gain a better understanding of site variability.
Construction of 45 liter reactor
To establish our first proof of concept, two 45 liter batch reactors were constructed to simulate the anaerobic and aerobic processes commonly found at treatment plants. Acrylic was chosen as the reactor material for its inert and transparent properties. The reactors were constructed with high length to diameter (L/D) ratios to let us observe the effects of settling, a phenomena distinctly observed in wastewater treatment as the population that survives (is recycled) is that which settles to the bottom of the secondary clarifier. Mixing was accomplished with an impeller, but required a relatively high torque for biosolids. The aerobic reactor was aerated with a single 9 inch ceramic aeration stone. Probes were used to monitor temperature, dissolved oxygen, and pH.
Acid Phase Digestion
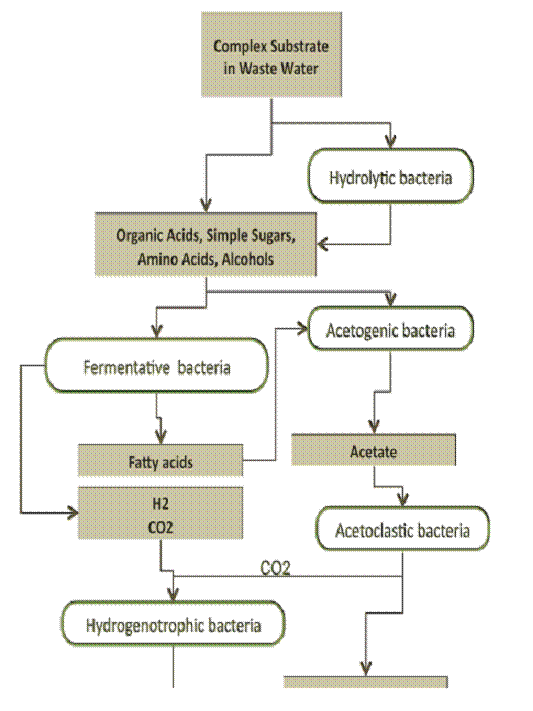
Compared to the process typically found at waste water treatment plants, digesting solids for fatty acid production requires similar but distinct processing conditions than those used for methane production. Methanogenesis, (biogas) production, necessitates that complex constituents are first broken into acids before conversion to gas, (see Fig. 1). The cascade of bioreactions that lead to methane includes a step of fatty acid production. This step typically peaks at a 2-5 day residence time and is occasionally used as a preliminary step to digestion where it is called acid phase digestion or APD. APD as a continuous acid production process achieves a steady state pH of 5.5-6 (lower pH is inhibiting) and a native population that self selects for acid production. Replicating this process as a batch requires a pH control mechanism and longer residence times given the un-optimized cell population. How this pH control is accomplished is important as many control systems will overshoot base addition, damaging the viability of some cell populations.
Figure 1: As described in Gerardi (2006) complex organic constituents are sequentially broken down ultimately to methane.
Sequence reactors, interesting results including some failures.
As expected, environmental factors dictate the nutrient consumption and storage rates (i.e. PHA production) of bacteria. Presently, there are three well-documented methods by which controlled environments are manipulated to favor the emergence and dominance of specific microbial species and biological behaviors (Lemos 2004, Din 2008). These three methods are the Feast Famine process where alternating feed and nutrient limitation cycles are imposed, Aerobic Dynamic Feeding (ADF) whereby feeding spikes are tied to changes in oxygen uptake of the organisms, and Aerobic/Anaerobic (AN/AE) cycling where aeration and thus dissolved oxygen is cycled.
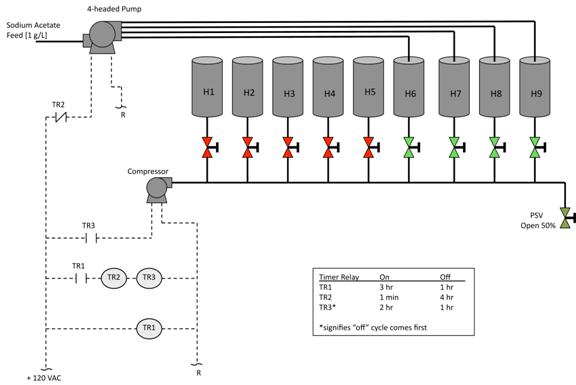
This project implemented an array of reactors that used combinations of Feast Famine and AN/AE cycling, (Fig 2). This system used nine constructed reactors that were linked to control timers that controlled aeration and nutrient cycling over different cycle times. The process feed was filtered digestor effluent supplemented with acetate. The results from these reactors varied dramatically across the array and over time. Some resultant populations demonstrated PHA per cell mass yields of over 30% for one given cycle and would then be overrun with fungal or protist infestation by the next cycle. After multiple
Figure 2: Equipment, reactor, and timer relay (TR) control diagram for feed and aerobic cycling of nine reactor array
To overcome this variability some of the productive species were cultured on solid nutrient media and run independently on synthetic broth. These resulting populations (species unknown) gave more consistent yields of more than 15% by dry cell mass when run within the 45 liter pilot reactor.
Difficulty of analysis,
The samples were analyzed for PHA content using gas chromatograph and a derivatization process similar to Braunegg et al. 1973 and Satoh et al. 1992. This analysis requires that the sample cells are no longer viable (or they consume the PHA bioplastic), that the depolymerase enzymes are no longer active, that the cells are lysed, and that a complete or known hydrolysis conversion occurs to give quantitative monomer peaks within the chromatogram. None of these issues is adequately addressed in literature. It was found that alkaline driven lysis can saponify ester bonds in PHA, bleach lysis is consistently incomplete, organic separation prevents water transfer and thus hydrolysis, propylated monomers give better partitioning than methylated monomers, and high derivatization or column temperature will produce some (but not complete) conversion to crotonic acid. Even as these variables that can either over or under represent the quantity of PHA in the sample, carefully run GC analysis was still found to be the most reliable of methods.
Extraction
Despite the chromatogram peak indicating reasonable production of PHA, an actual chemical extraction of plastic from a reactor was wanted for proof of concept. As seen with the analysis effort, standard extraction methods were also difficult to implement. A standard lysis solution contains anionic surfactant(s) to disrupt cellular membranes. This same surfactant emulsifies traditional two phase organic/aqueous extraction systems. To overcome this emulsion a combination of salting, heat, and centrifugation was used to give complete separation of phases. The organic phase was then dosed with an excess of cold methanol anti-solvent to drop the plastic out of solution without drying large amounts of solvent. More than 70% of the predicted PHA content was recovered using this method.