You are here:
EMISSIONS REDUCTION OF COMMERCIAL GLASSMAKING USING SELECTIVE BATCHING
Impact/Purpose:
Selective batching involves separating a glass batch into two or more, chemically unique mixtures. When the two mixtures are added together, they yield the final batch chemistry. Selective batching technologies offer a substantial opportunity to close the gap between theoretical and practical glass melting energy consumption.
Selective batching controls reaction paths to avoid a eutectic liquid forming between sodium carbonate and calcium carbonate. In conventionally batched melts, this leads to high temperature melt segregation. Batch free time reductions (up to 80%) have been achieved using selective batching. Batch free time is defined as the amount of time a glass batch takes to complete the dissolution of all batch ingredients.
While increased efficiency has been observed, the fining behavior of selectively batched melts has yet to be investigated. Fining refers to the removal of bubbles present in the glass as undissolved gas, and implies a homogenous glass. It is proposed that selective batching can improve on fining in two ways (1) by yielding a developing melt whose viscosity is more consistent and higher during the melting process, and (2) by tailoring the size of the voids between granules in a selective batch to achieve fast fining by enhanced Stokes’ fining.
Description:
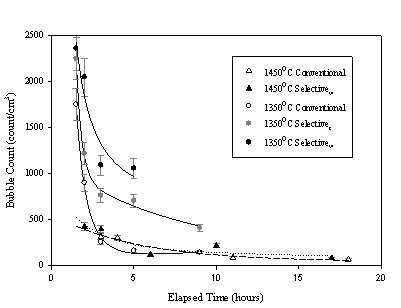
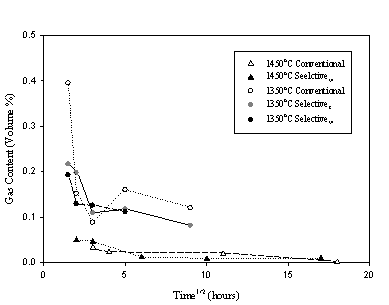
The vertical bubble populations of selectively batched melts were compared to the vertical bubble populations of conventionally batched melts. “Conventional” refers to the use of a powdered batch. Bubble position and diameter measurements were taken on 24 crucibles encompassing five trials. The glass composition studied was a generic float glass (Table I). Three melts at 1350 °C revealed distinguishable differences in bubble diameter, bubble concentration, and gas volume percent results between conventional and selective trials (Figures 1-3).
Table I. Glass Composition Studied.
|
Oxide |
Mol % |
Weight % |
|
SiO2 |
71.9 |
73.0 |
|
Na2O |
13.2 |
13.0 |
|
CaO |
10.5 |
10.2 |
|
MgO |
4.3 |
3.0 |
|
K2O |
0.13 |
0.2 |
|
Al2O3 |
0.06 |
0.1 |
Figure 1. Mean bubble diameter of the distribution as a function of melting time. The conventional trial shows the largest mean bubble diameters throughout the time scale shown, while the Selectivet trial has larger mean bubbles than the Selectivew trial.
Figure 2. Bubble concentration (N) per cubic centimeter of glass. The Conventional trial at 1350 °C has the fewest number of bubbles throughout the time scale measured.
Figure 3. The (undissolved) gas content for each melting trial is displayed and compared against one another. The lines between symbols are a guide for the eye.
The selectively batched trials contained a higher concentration of bubbles than conventionally batched melts. The mean bubble diameter was smaller in selectively batched melts than conventionally batched melts. This means more, smaller bubbles.
Vertical bubble position versus bubble diameter were plotted as an alternative, static approach for evaluating fining behavior. The plots revealed that conventionally batched melts contain significant (up to one third of the melt depth) regions with few to no bubbles while selectively batched melts all exhibited consistent vertical bubble distributions.
In response to these findings, the viscosity of the melts was calculated using the Vogel-Fulcher-Tammann Equation. The calculation was made twice for each sample, using two sets of data. Hot stage microscopy and dilatometry measurements were used to arrive at one set of VFT parameters, while chemical analysis data were used to arrive at another (Table II).
Table II. Calculated Viscosity Results for Samples Melted at 1350 °C for 5 hours
|
Sample |
Method |
|
|
HSM/Dilatometer (Pa*s) |
ICP (Pa*s) |
|
|
Conventional |
103.65 |
101.28 |
|
Selective, truncated GSD |
102.52 |
101.37 |
|
Selective, wide GSD |
102.84 |
101.33 |
The abnormally high viscosity result for the conventional trial measured using HSM and dilatometry can be explained by sample preparation differences. HSM and chemical analysis samples are powdered glass and are therefore more homogenized when compared to the bulk glass. The dilatometer samples are not homogenized and represent the true homogeneity of the bulk glass. The Selectively batched melts return consistent results because selective batching results in a more consistent developing viscosity profile.
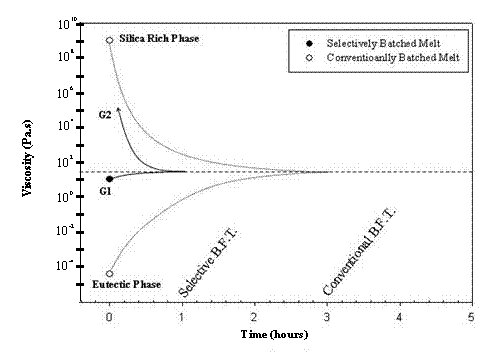
Selectively batched melts contain a consistent, vertical bubble population as a result of two granules with two starting viscosities that are much higher than the viscosity of the eutectic liquid formed in a segregated melt (Figure 4). The low viscosity liquid in a selectively batched melt (granule #1) is on the same order of magnitude as the viscosity of the final melt. The starting viscosities in a selectively batched melt are also closer together than the viscosities of the two regions in a segregated melt.
Figure 4. Proposed dynamic viscosity model of conventionally and selectively batched melts at 1350 °C. Each melt is assumed to have two starting viscosity reference points. G2’s viscosity is undefined at the melting temperature of 1350 °C.